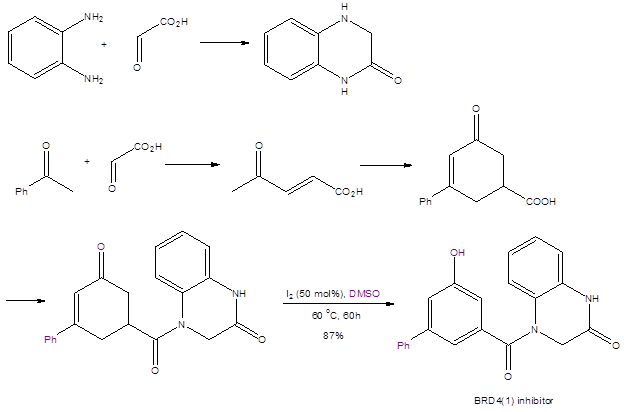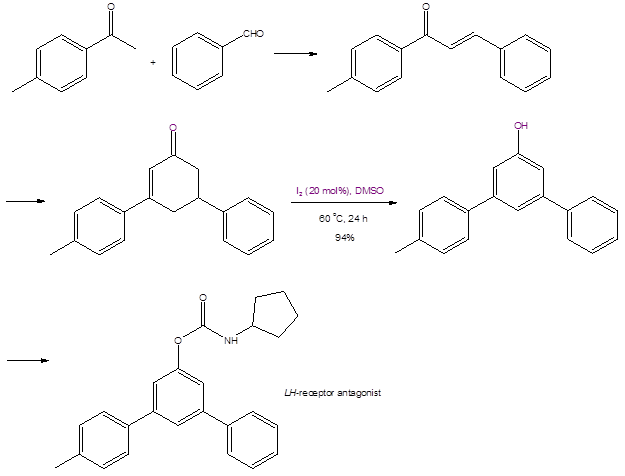A recent Synthesis Corner post (March, 2017, “DMSO/I2 Catalyzed Synthesis of Catechols from Cyclohexanones”) reviewed work where cyclohexanones acted as convenient/cheap starting materials for the synthesis of aromatic compounds through a DMSO/I2 catalyzed route.1 Another recent article by Luo and co-workers also demonstrates the usefulness of the DMSO/I2 catalyst system to produce aromatic compounds from nonaromatic starting materials.2 In this case, Luo’s group explores the synthesis of a variety of phenols from various substituted cyclohexenones (Equation 1).
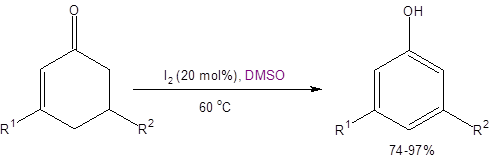
Equation 1: DMSO/I2 synthesis of substituted phenols from cyclohexenones
While substituted phenols are valuable precursors in several synthetic schemes, traditionally routes to these compounds have relied on functionalization of preexisting aromatic rings through electrophilic and/or nucleophilic aromatic substitution. The preparation of meta-substituted phenols proves to be especially challenging due to the usual mismatch of the desired regiochemistry and the inherent directing effects of substituents. This usually means a tedious multi-step approach to achieve the proper substituent placement.
To avoid the difficulties with traditional routes, some groups have turned to metal-catalyzed oxidative aromatization schemes to synthesize meta-substituted phenols.3-7 This too comes with its own challenges: 1) the tendency to overoxidation, and 2) the necessity of an excess of the halogen oxidant.
Therefore, the reaction shown in Equation 1 has many advantages not seen with either the traditional aromatic substitution route or the metal-catalyzed oxidative aromatization route. The DMSO/I2 route provides direct access to meta-substituted phenols using widely available, inexpensive, and low toxicity materials. It also avoids metal catalysts and unwanted overoxidation.
The reaction shown in Equation 1 is tolerant of electron-withdrawing and electron-donating groups at either R1 or R2. Notably, the reaction is even successful when R2 is a carboxylic acid, ester or amide functionality, thereby making meta-hydroxybenzoic acids, -esters, and -amides. Additionally, the method is tolerant of R1 and R2 being or containing haloaryls, acetals, furanyls, Boc-protected amines, nitros, and p-phenols.
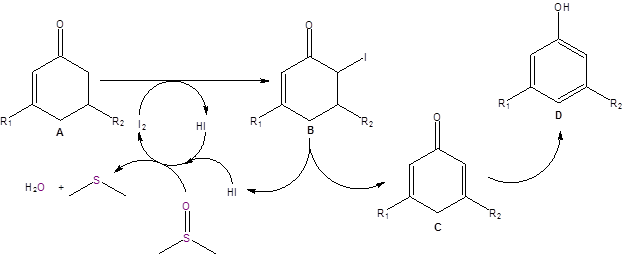
Mechanistic studies indicated that substituents at position 6 on the ring resulted in poor yields of the phenol. Additionally, the reaction failed with KI, and NXS was less efficient that molecular I2. All solvents other than DMSO either resulted in no reaction or a complicated mixture. With this information and with comparison with other proposed DMSO/I2 mechanisms, the authors proposed the following mechanistic pathway (Scheme 1).
Scheme 1: Proposed mechanism.
In this proposed mechanism, the enone A undergoes iodination at the alpha carbon 6 to produce intermediate B. Intermediate B undergoes loss of HI to produce C followed by a tautomerization to produce the phenol D. As shown in the mechanism, the iodide ions are oxidized back to molecular iodine by DMSO. Therefore, DMSO not only acts as the solvent but also as the terminal oxidant for the reaction.
This synthetic method has been used to synthesize two bioactive molecules. The first de novo synthesis of the BRD4(1) inhibitor shown in Scheme 2 features the DMSO/I2 reaction in the last step. This synthetic method has high yields in all steps and uses only readily-available and cheap starting materials.
Scheme 2: The de novo synthesis of a BRD4(1) inhibitor.
The synthesis of another bioactive molecule, an LH-receptor antagonist (Scheme 3), was prepared through a de novo synthetic scheme featuring the DMSO/I2 aromatization reaction (the penultimate step). Again, this synthetic scheme uses readily-available and inexpensive materials, and all of the steps give excellent yields.
Scheme 3: The de novo synthesis of an LH-receptor antagonist
In conclusion, the Luo group’s work demonstrates another valuable use of the DMSO/I2 catalytic system in the synthesis of aromatic compounds. The reaction is metal-free, provides ready access to meta-substituted phenols in high yields, uses only inexpensive, readily-available materials of low toxicity, and has broad functional group tolerance.
(1) Liang, Y.-F.; Li, X.; Wang, X.; Zou, M.; Tang, C.; Liang, Y.; Song, S.; Jiao, N. Journal of the American Chemical Society 2016, 138, 12271.
(2) Wang, S.-K.; Chen, M.-T.; Zhao, D.-Y.; You, X.; Luo, Q.-L. Advanced Synthesis & Catalysis 2016, 358, 4093.
(3) Izawa, Y.; Pun, D.; Stahl, S. S. Science 2011, 333, 209.
(4) Imahori, T.; Tokuda, T.; Taguchi, T.; Takahata, H. Organic Letters 2012, 14, 1172.
(5) Pun, D.; Diao, T.; Stahl, S. S. Journal of the American Chemical Society 2013, 135, 8213.
(6) Zhang, J.; Jiang, Q.; Yang, D.; Zhao, X.; Dong, Y.; Liu, R. Chemical Science 2015, 6, 4674.
(7) Zhang, Z.; Hashiguchi, T.; Ishida, T.; Hamasaki, A.; Honma, T.; Ohashi, H.; Yokoyama, T.; Tokunaga, M. Organic Chemistry Frontiers 2015, 2, 654.