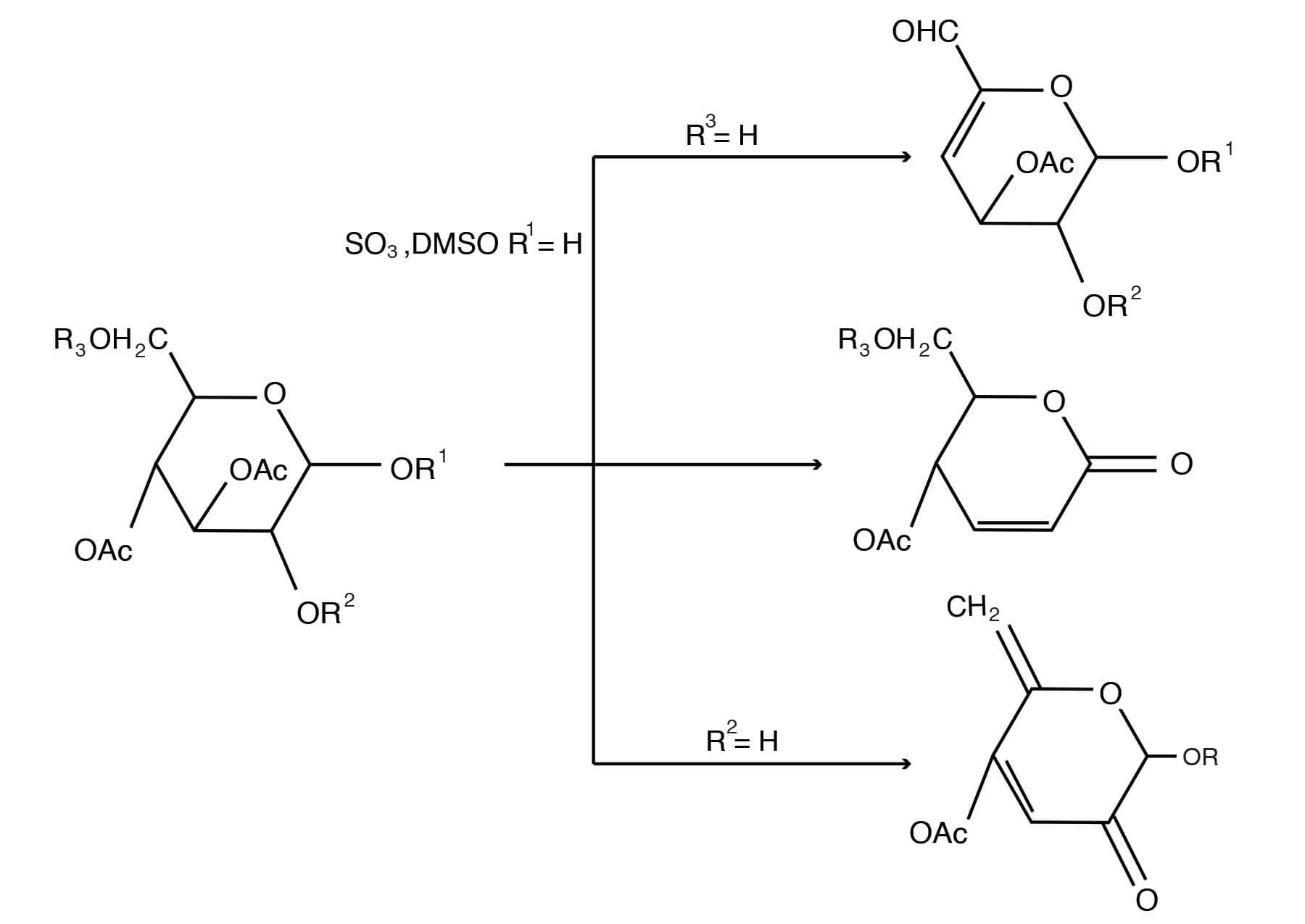
The combination of DMSO with S03-pyridine complex in the presence of triethylamine yields a reagent that rapidly oxidizes primary and secondary alcohols in good yield at room temperature to aldehydes and ketones, respectively [Omura, K., Swern, D., Tetrahedron 34, 1651-1660 (1978); Mancuso, A. J., Swern, D., Synthesis, 165-185 (1981)]. An attractive feature of this reagent is its property of effecting oxidation of allylic alcohols to the corresponding a, ß-unsaturated carbonyl compounds [Parikh, J. R., J. Am. Chem. Soc. 89 (21), 5505-5507 (1967)]. The S03-pyridine complex in DMSO can be used to oxidize acid-labile trans-diols [Marshall, J. A., Cohen, G. M., J. Org. Chem. 36, 877-882 (1971)] or cis-diols [Harvey, R. G.; Goh, S. H.; Cortez, C., J. Am. Chem. Soc. 97, 3468-3479(1975)] to quinones. This reagent has also been used to oxidize alkaloid hydroxyl groups to ketone groups [Milborrow, B. V., Djerassi, C., J. Chem. Soc. C 417-424 (1969); Chinn, L. J., Desai, B. N., Zawadzki, J. F., J. Org. Chem.40, 1328-1331 (1975)]. Application of the DMSO-S03 pyridine reagent to partially acetylated carbohydrates leads to oxidation as well as elimination of the elements of acetic acid, thus providing a high yield to novel unsaturated carbohydrates [Cree, G. M., Mackie, D. W., Perlin, A. S., Can. J. Chem. 47, 511-512 (1969)].
Oxidation Formula: