IBX / DMSO
The report of Frigerio and Santagostino (1) that 2-iodoxybenzoic acid is an efficient and mild alcohol oxidant in DMSO was initially published in 1994.
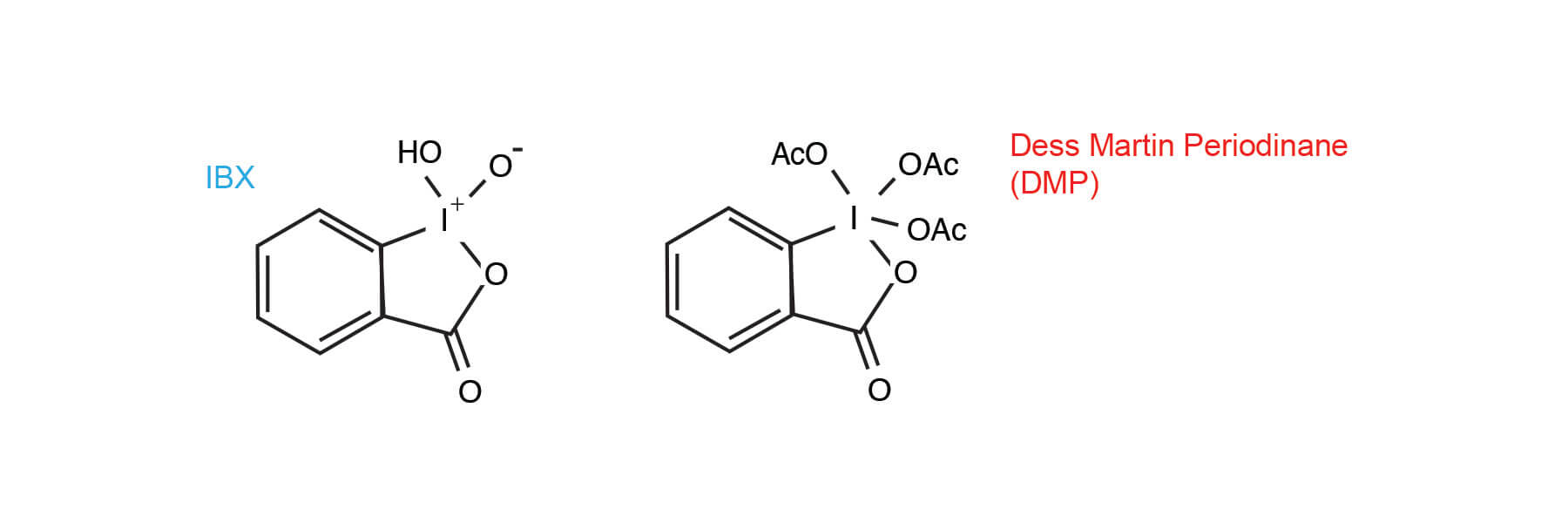
An analogue of the previously reported 12-I-5 Dess-Martin Periodinane, the IBX / DMSO reagent has some attractive features as an oxidant. It is:
- Cheap to prepare from readily available reagents (viz. an oxidant such as KMnO4, Oxone, KBrO3 or Cl2is reacted with 2-iodobenzoic acid). (8)
- More selective than many other oxidants. To this point, alcohols can be oxidized in the presence of thioethers and amines (3) ; 1,4-diols can be converted to γ-lactols without overoxidation to the lactone (4). Olefins, carboxylic acids, amides, and esters are all unaffected by IBX exposure (5).
- Effectively used under mild conditions. IBX / DMSO reactions are typically performed at room temperature and are complete in a few hours. It is worthwhile mentioning the fact that the very low temperatures often required by most ‘activated DMSO’ reactions (i.e. the oxalyl chloride / DMSO ‘Swern’ oxidation) are not needed. This recommends the use of IBX / DMSO for large-scale oxidations.
- Easily handled and used. In contrast to DMP, IBX / DMSO is unaffected by moisture and can be used in the open air (2).
IBX has an interesting history and was largely ignored for over 100 years after its discovery by Hartman and Meyer (6). The poor solubility of IBX in almost all solvents seemed to preclude its usefulness until the 1994 report by Santagostino that solutions as high as 1.5 M can be prepared in DMSO. Until that time IBX was employed as the precursor to the Dess-Martin periodinane (DMP) reagent (7).
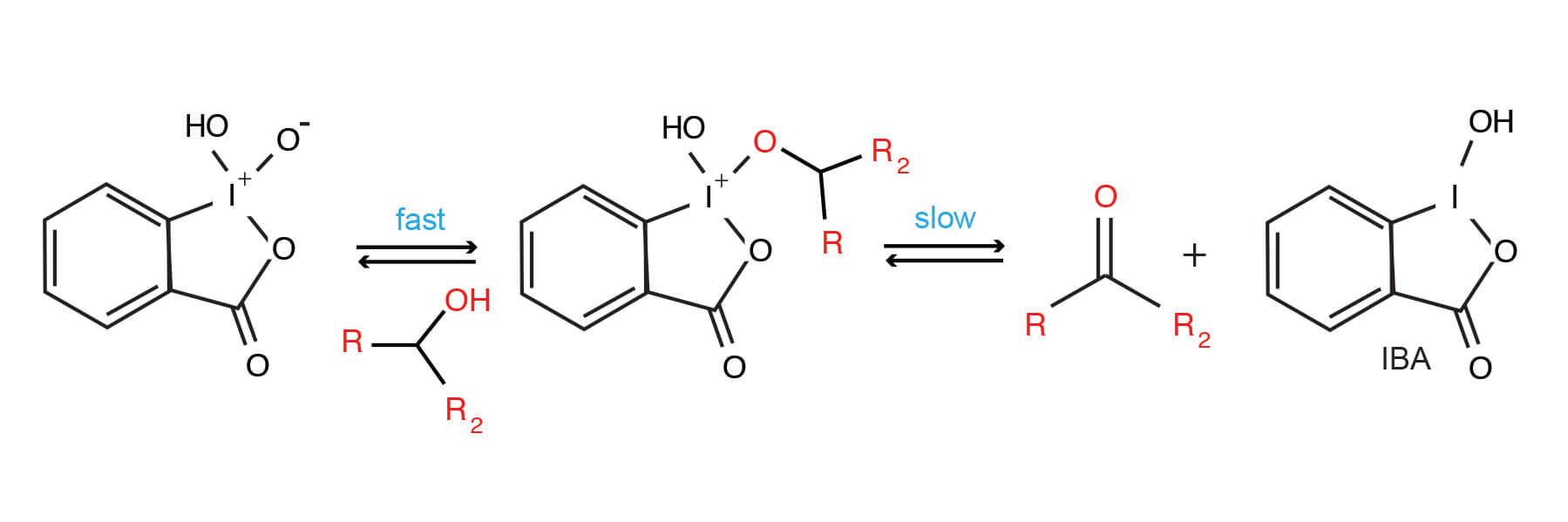
Although the general mechanism of IBX/DMSO alcohol oxidation has been described, there are some curious aspects associated with this reagent. Some evidence exists that the reactivity of IBX is altered when solvent molecules are incorporated as ligands (9).
Additionally it has been shown that IBX can serve as an effective oxidant in suspension with organic solvents other than DMSO (10). Although IBX can exist in two crystal forms, it seems that both serve equally well as oxidizing agents in suspension. Finney et al. have described a variation of the IBX oxidation which simplifies workup by using the reagent in relatively poor solvents at elevated temperatures (14)
Generalized Mechanism of IBX-mediated alcohol oxidation.
IBX can be explosive; its tendency to explode is apparently related to its purity (11). Wet IBX samples have reportedly explode at above 130°C (12). A US patent claims that mixtures of IBX, benzoic and isopthalic acids will not explode. This safer reagent has been termed ‘SIBX’ (13).
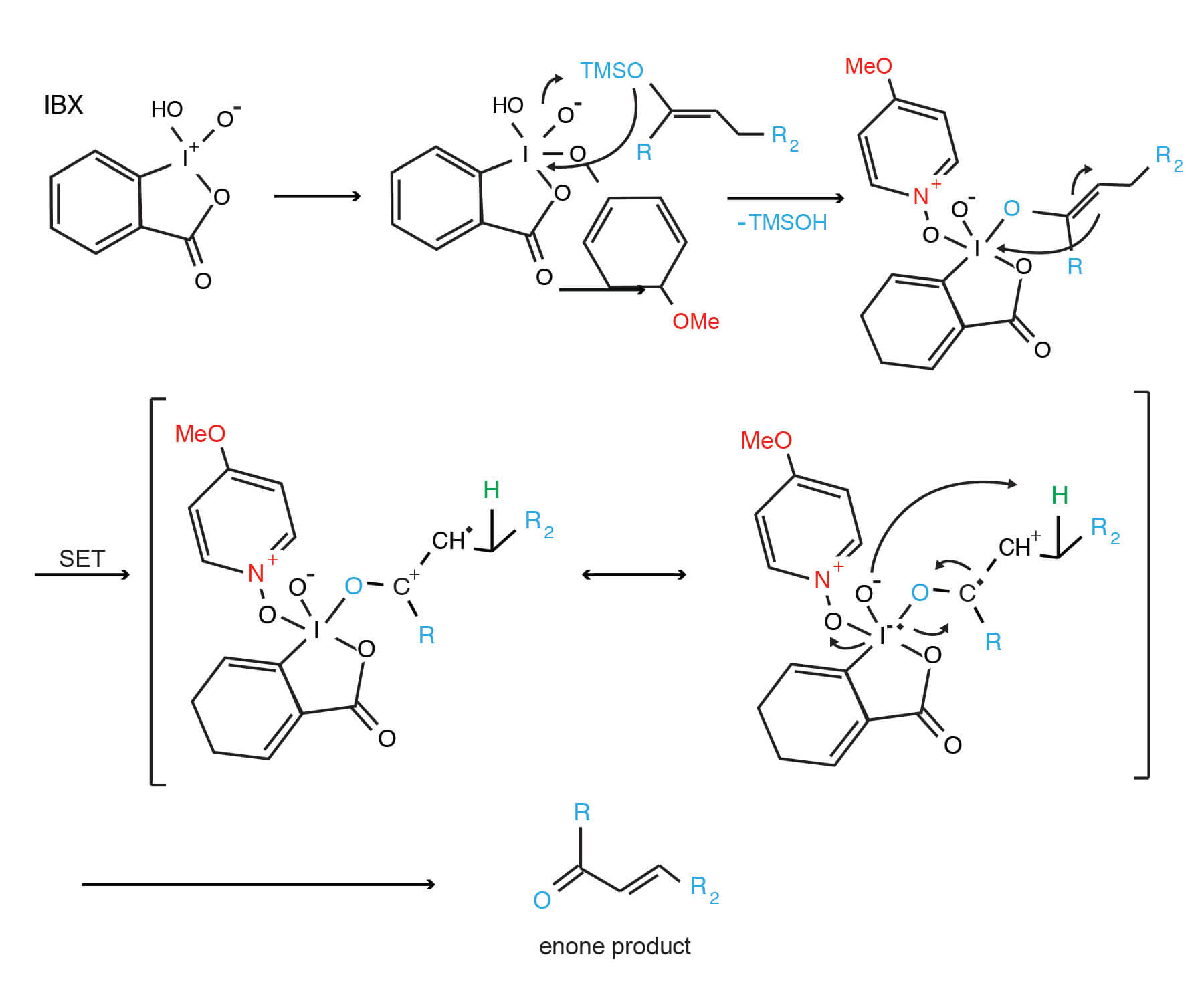
An interesting use of the IBX/DMSO reagent is highlighted in the Synthesis Workbook III (14). Reaction of IBX with TMS enol ethers provides enones via the mechanism proposed by K.C. Nicolaou.
IBX oxidation of silyl enol ethers: a novel enone synthesis (14)
Click to enlarge for easier viewing.
Variations of the IBX reagent have been devised which are functionalized to make them water soluble (15). Such derivatives are useful for the smooth and and chemoselective oxidation of allylic and benzylic alcohols in water without overoxidation. Resin-bound and silica-supported IBX-type reagents have been published which can be used to simply reaction workup. (16)
Example of an IBX oxidation (17).
Alcohol is combined with a 0.4-1.0 M solution of IBX in DMSO. A molar excess of IBX is recommended of between 1-10 equivalents. When amines are present an acid such as TFA (1-1.5 equivalents) are prescribed. Addition of water, filtration of the precipitate, and extraction into an appropriate water immiscible extraction solvent comprises a typical workup procedure. A adapted procedure from the 1994 work of Frigerio et al (1) follows. IBX (1.9 mmol) was added to a DMSO (5 mL) solution of the alcohol (1.32 mmol). After stirring for 3.5 hours water was added (20 mL) and the mixture was filtered. Extraction into ether (3 x 50 mL), drying of the ether extract with Na2SO4, and concentration provided a crude product residue which was purified by flash chromatography. The ketone product was obtained in 86% isolated yield.References
(1) Frigerio, M. ; Santagostino, M.; Sputore, S.; Palmisano, G. J. Org. Chem. 60 (1995) 7272-7276.
(2) The presence of water in the reaction does typically retard reaction rates.
(3) Frigerio, M. ; Santagostino, M.; Sputore, S.; Palmisano, G. J. Org. Chem. 60 (1995) 7272-7276.
(4) Corey, E.J.; Palani, A. Tetrahedron Lett. 36 (1995), 3485.
(5) Frigerio, M. ; Santagostino, M. Tetrahedron Lett. 35 (1994), 8019.
(6) Hartmann, C.; Meyer, V. Chem. Ber. 26 (1893), 1727.
(7) Dess, D.B.; Martin, J.C. J. Org. Chem. 48 (1983), 4155.
(8) Katritsky, A.R.; Duell, B.L.; Gallos, J.K. Org. Magn. Reson. 27 (1989) 1007; Hartmann, C.; Meyer, V. Chem. Ber. 26 (1893), 1727.; Dess, D.B.; Martin, J.C. J. Org. Chem. 48 (1983), 4155.
(9) Nicolaou, K.C.; Montagnon, T.; Baran, P.S. Angew. Chem. Int;. Eng. Ed. 41 (2002), 993.
(10) More, J.D.; Finney, N.S. Org. Lett. 4 (2002), 3001
(11) Boeckmann Jr, R.K.; Shao, P.; Mullins, J.J. Org. Synth. 77 (2000), 141.
(12) Dess, D.B.; Martin, J.C.; J. Am. Chem. Soc. 113 (1991), 7277.
(13) Depernet, D.; Francois, B.; Ozanne, A.; Pouységu,L. ; Quideau, S. Org. Lett., 2003, 5 (16), pp 2903–2906
(14) Kinzel, T.; Major, F.; Raith, C.; Redert, T.; Stecker. F.; Tölle, N.; zinngrebe, J. Organic Synthesis Workbook III Wiley-VCH pubs. (2007) 158-159.
(15) Thottumkara, A.P.; Vinod, T.K. Tetrahedron Lett. 43 (2002) 569.
(16) Sorg, G.; Mengel, A.; Jung, G.; Rademann, J. Angew. chem. Int. Ed. Eng. 41 (2001) 4395.
(17) Tojo, G.; Fernández, M. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice Springer, Pubs. (2006) 205
ASM recommends the excellent chapter on the subject of hypervalent iodine oxidants in Tojo and Fernández book (reference 17). Much of the general materials above was related in Chapter 3.3, pages 202-211.