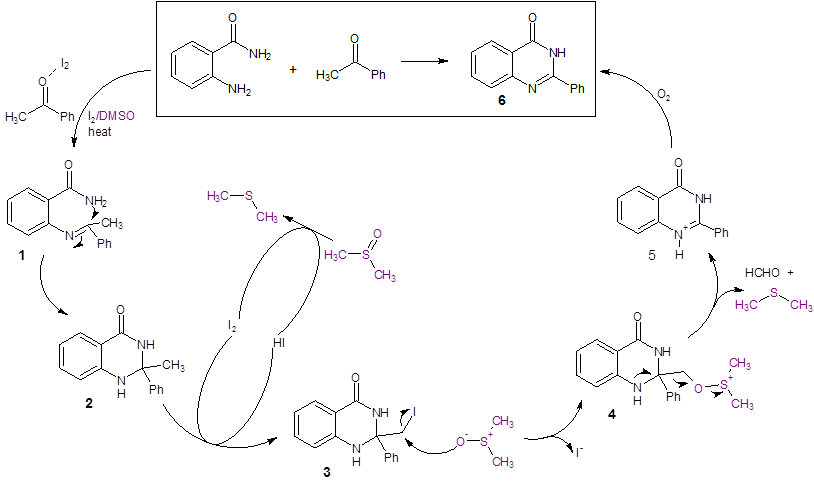
A recent report by Vishwakarma and Bharate in the Journal of Organic Chemistry establishes a metal-free route to quinazolinones and pyrazolo-pyrimidinones using a DMSO/I2 catalytic system (Equation 1).1
DMSO acts as more than just a solvent in the reaction—it serves as an active participant in the reaction mechanism.
DMSO/I2 catalytic systems have been used previously in the synthesis of quinazolinones,2-4 and in some instances they prove to be viable alternatives to using transition metal catalysts.
As quinazolinones and pyrazolo[4,3-d]pyrimidin-7(6H)-ones are medicinally important, there has been much work exploring routes to these fused heterocycles in recent years.2-9 In fact, a recent post demonstrates another route to quinazolinones employing DMSO (March, 2016 Synthesis Corner post).10
The method described in the Vishwakarma and Bharate article has advantages over many other known routes in that 1) it doesn’t require stoichiometric amounts of oxidants or coupling agents;5 2) there is no need for metal catalysts;6-9 3) the starting materials are relatively cheap and readily available; and 4) it only requires catalytic amounts of iodine and molecular oxygen.
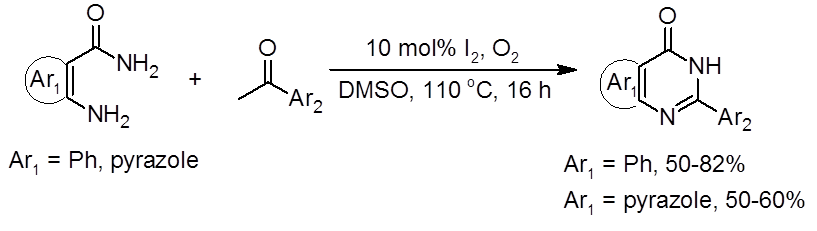
Equation 1: DMSO/I2 route to quinazolinones and pyrazolo[4,3-d]pyrimidin-7 (6H)-ones
The drug sildenafil (Viagra) contains a pyrazolo[4,3-d]pyrimidin-7(6H)-one pharmacophore, and this paper describes using the above method to synthesize a key intermediate in the synthesis of sildenafil (55% yield). In addition, the authors report the synthesis of other molecules of pharmaceutical interest such as an intermediate used in the synthesis of a potent mTOR inhibitor (Figure 1).
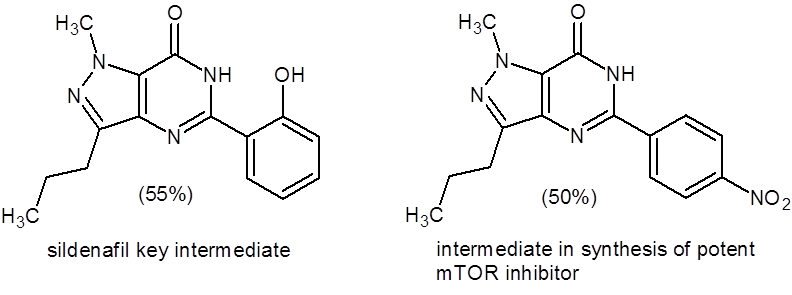
Figure 1: Important pyrazolo-pyrimidinones synthesized by the method outlined in Equation 1.
As stated previously, DMSO actively participates in the reaction, so it cannot be exchanged for other solvents. In the proposed mechanism (Scheme 1), iodine activates acetophenone so that the imine intermediate 1 readily forms. This undergoes cyclization to form intermediate 2. The methyl carbon in 2 is iodinated by I2 to form intermediate 3. DMSO oxidizes I– back to I2 in a separate cycle. DMSO acts to displace I– from intermediate 3 to form 4. Intermediate 4 eliminates formaldehyde and dimethyl sulfide to form 5 which is the protonated form of the product 6.
Scheme 1: Proposed Mechanism
Of note, while some of the products were purified by column chromatography, most were purified by the following method: Methanol was added to the crude product, and the mixture was allowed to sit for 2-3 days to form a suspension. On filtering, the residue was washed with methanol 2-3 times and dried to produce crystalline pure product.
These researchers have described a new DMSO/I2 catalyzed synthesis of medicinally-important fused heterocycles. This route’s utility has been demonstrated in synthesizing a key intermediate in the production of sildenafil. The method uses readily-available starting materials and requires only standard reaction set up and a simple work up.
Debra Dolliver, Ph.D.
References
(1) Mohammed, S.; Vishwakarma, R. A.; Bharate, S. B. J. Org. Chem. 2015, 80, 6915.
(2) Ge, W.; Zhu, X.; Wei, Y. RSC Adv. 2013, 3, 10817.
(3) Nguyen, T. B.; Ermolenko, L.; Al-Mourabit, A. Green Chem. 2013, 15, 2713.
(4) Wei, H.; Li, T.; Zhou, Y.; Zhou, L.; Zeng, Q. Synthesis 2013, 45, 3349.
(5) Mitobe, Y.; Ito, S.; Mizutani, T.; Nagase, T.; Sato, N.; Tokita, S. Bioorg. Med. Chem. Lett. 2009, 19, 4075.
(6) Hikawa, H.; Ino, Y.; Suzuki, H.; Yokoyama, Y. J. Org. Chem. 2012, 77, 7046.
(7) Zhou, J.; Fang, J. J. Org. Chem. 2011, 76, 7730.
(8) Sharif, M.; Opalach, J.; Langer, P.; Beller, M.; Wu, X.-F. RSC Adv. 2014, 4, 8.
(9) Jiang, X.; Tang, T.; Wang, J.-M.; Chen, Z.; Zhu, Y.-M.; Ji, S.-J. J. Org. Chem. 2014, 79, 5082.
(10) Kim, N. Y.; Cheon, C.-H. Tetrahedron Lett. 2014, 55, 2340.