Fluorinated intermediates and active pharmaceutical ingredients (APIs) are of considerable importance in the pharmaceutical industry since about 10% of all APIs are fluorinated. For the past 20 years, fluorinated intermediates have known a great success in Life Science actives, as the introduction of one or several fluorine atoms can increase a drug’s biological activity (Rhodia, “Perfumery, Performance, & Agro”, July, 2004).
One of the best and least expensive ways to introduce fluorine into a molecule is via the Halex reaction. In this context, the Halex reaction involves the nucleophilic aromatic substitution of fluoride ion for chloride or bromide in electron-poor aromatic systems. Such systems may contain heterocyclic nitrogen, e.g. pyridines or may contain electron-withdrawing groups such as aldehydes nitro-,or cyano-. . Exchange reactions between halogens often require high temperatures and very polar solvents.
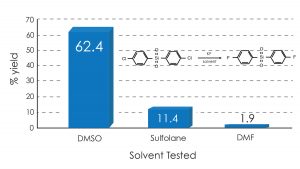
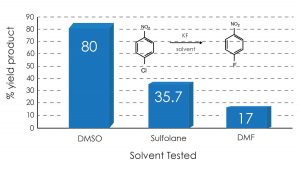
In the examples shown below, the surprising finding is that DMSO use gave dramatically higher yields in the Halex reaction of p-nitrochlorobenzene and bis(p-chlorophenyl) sulfone with anhydrous potassium fluoride compared with traditional solvents such as DMF and sulfolane.