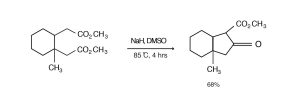
The Dieckmann condensation of 1,2-bis(carboxymethyl)-3-methylcyclohexane dimethyl ester with sodium hydride in DMSO furnishes a crystalline keto-ester [Coates, R. M.; Chung, S. K., J. Org. Chem. 38, 3740-3741 (1973)].
Our laboratory work has employed the dimsyl ion in a similar reaction resulting in enhancing the product yield to a near-quantitative level.
Reaction of diethyl γ-ketopimelate with an excess of methylenetriphenylphosphorane in DMSO provides for the introduction of an exocyclic methylene group and subsequent Dieckmann condensation to the carbethoxycyclohexanone [Matcha, R. L., J. Am. Chem. Soc. 95, 7508-7510 (1973)].