Introduction to DMSO Physical Properties
Dimethyl Sulfoxide (DMSO) is a highly polar and water miscible organic liquid. It is essentially odorless, and has a low level of toxicity. DMSO is a dipolar aprotic solvent, and has a relatively high boiling point. Further below is a compilation of Physical Properties data, such as DMSO density and stability, for this useful solvent.
Contents
- Typical DMSO Properties
- Vapor Pressure vs. Temperature Curve for DMSO
- Specific Gravity of DMSO as a Function of Temperature
- DMSO Viscosity as a Function of Temperature
- Initial Sorption Rates of DMSO at Various Relative Humidities
- Hygroscopicities of DMSO at Various Relative Humidities at 22°C
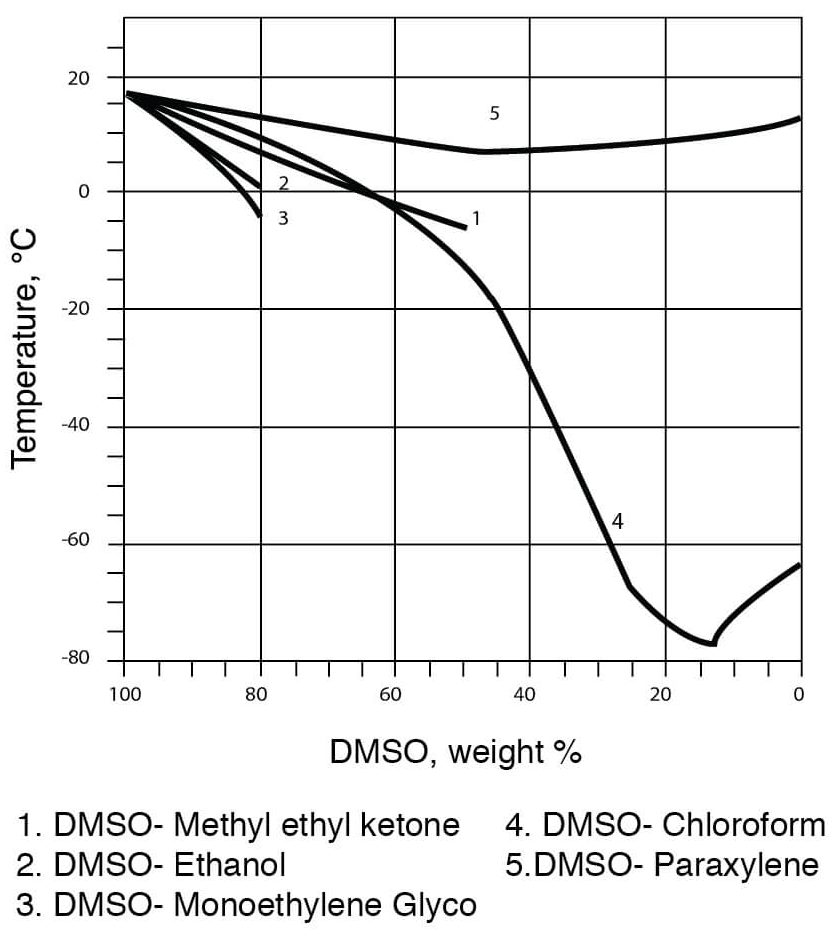
- Freezing Temperatures for DMSO – Water Solutions
- Freezing Point Data for DMSO – Water Solutions
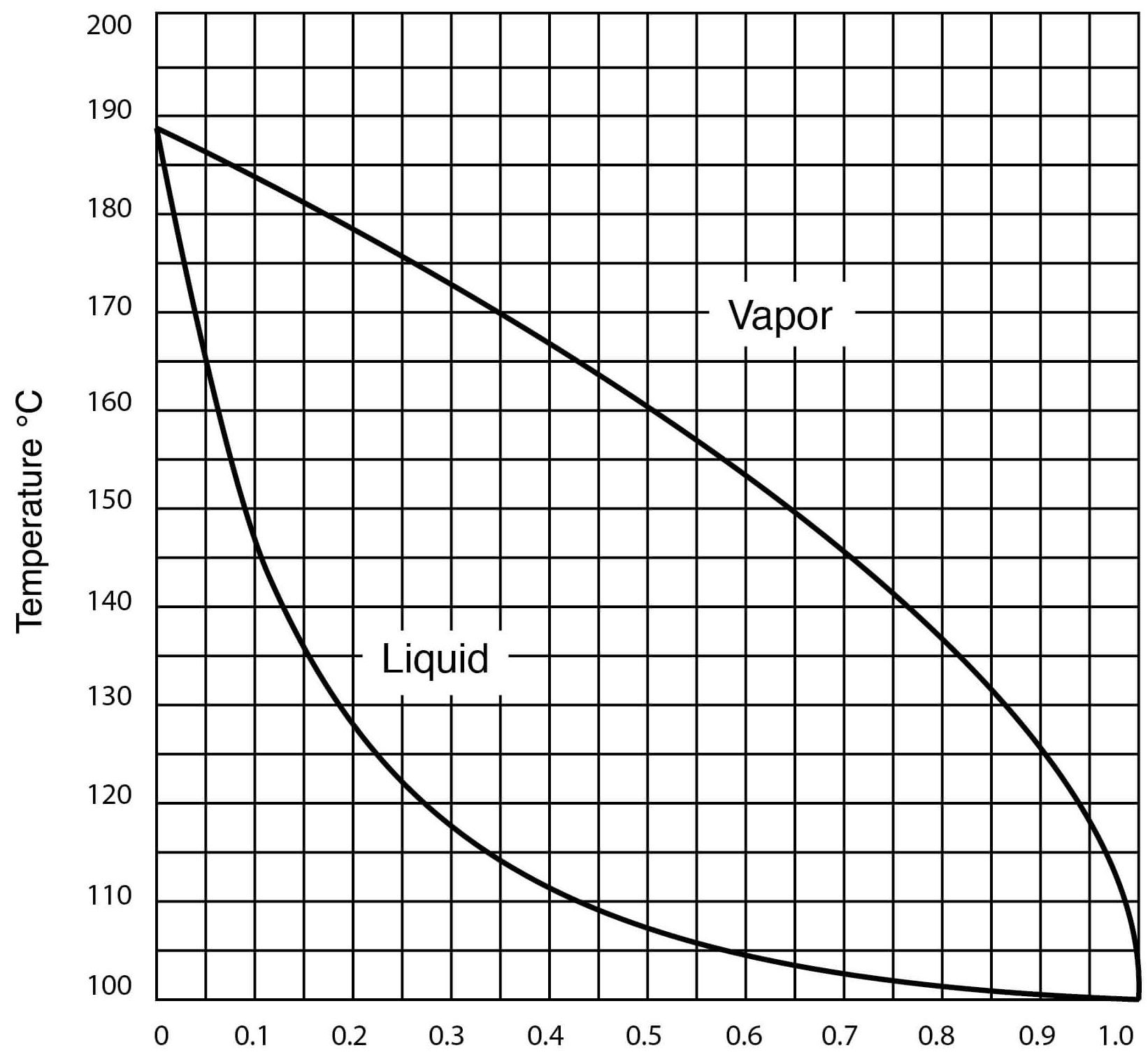
- Vapor – Liquid Equilibrium for DMSO – Water Solutions
- Heat Capacity and Density of DMSO
- Boiling Point/Temperature Curves: DMSO – Water Solutions
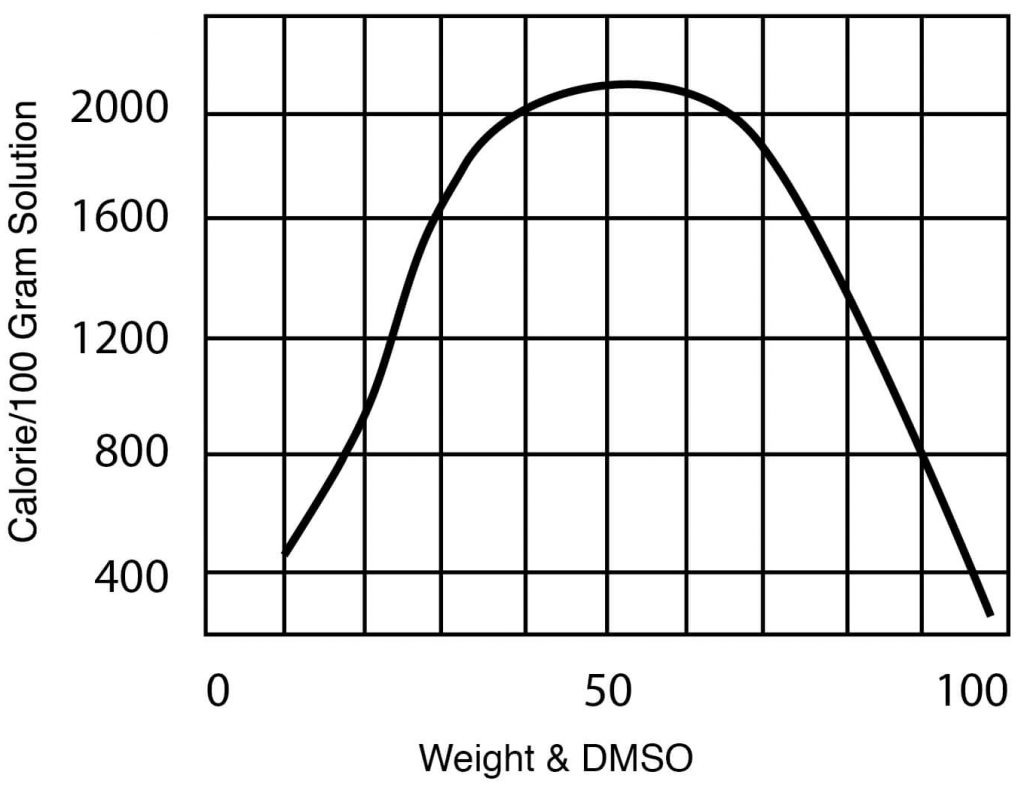
- Heat of Mixing, DMSO – Water mixtures, 32°C
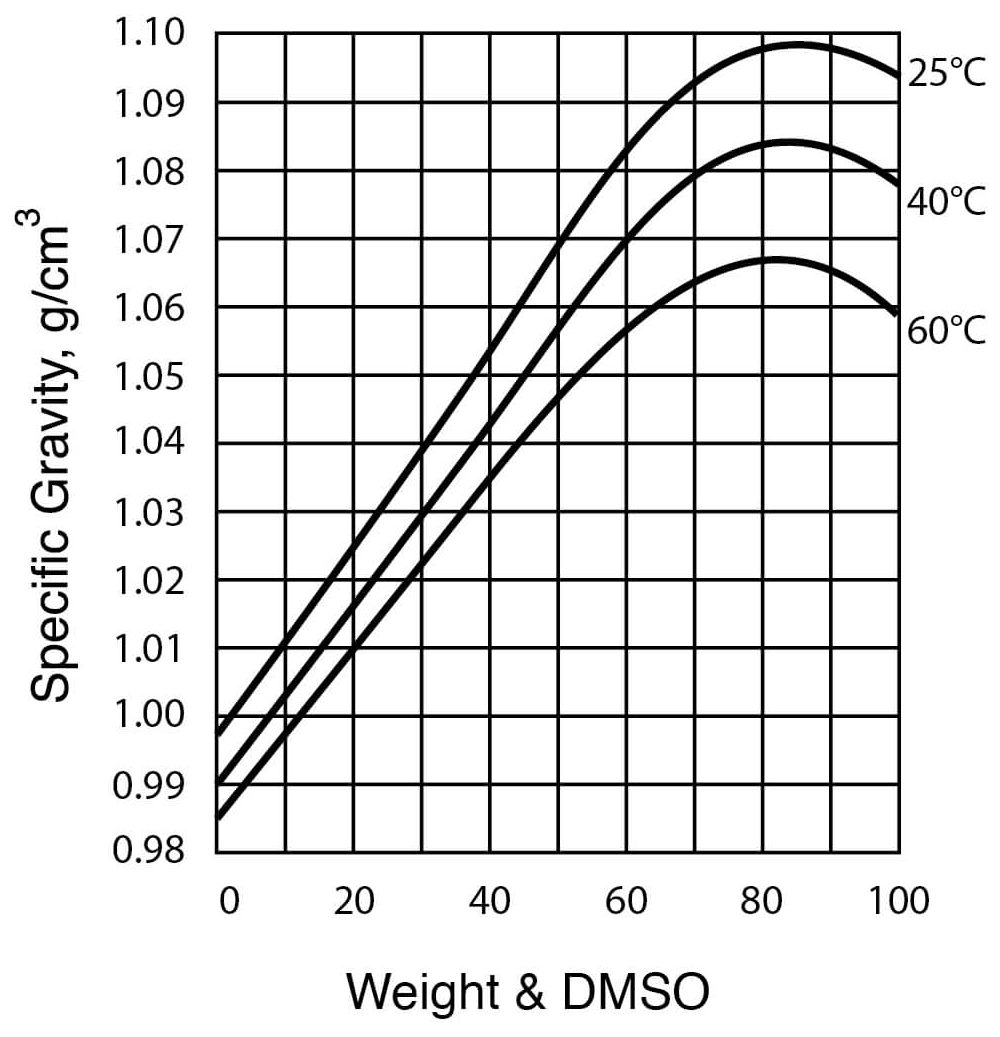
- Specific Gravity of DMSO – Water Solutions
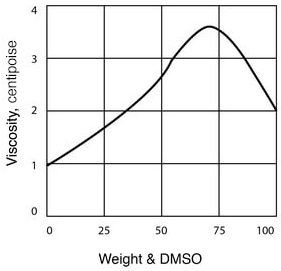
- Viscosity of DMSO – Water Solutions
- Chemical and Thermal Stability of DMSO
- Comparative Viscosities and Evaporation Times of Common Solvents
| Parameter | Value |
| Auto ignition temp, in air | 300-302°C (572-575°) |
| Boiling point (1 atmosphere) | 189°C (372°F) |
| Coefficient of expansion | 0.00088/°C |
| Conductivity (Electrical), 20°C 3×10-8 (ohm-1 cm-1) | 3 x 10-8 (ohm-1 cm-1) |
| @ 80°C | 7 x 10-8 (ohm-1 cm-1) |
| Critical heat flux (4.10×105 J / s / m2) | 1.3 x 105 Btu/hr x ft-2 |
| Critical molar volume | 2.38 x 10-4 m3 |
| Critical Pressure | 56.3 atm. abs. |
| Critical temperature | 447°C (837°F) |
| Density, at 25°C (see Figure 3) | 1.0955 g / cm3 |
| Dielectric constant, 1 MHz, @ 20°C | 48.9 |
| @ 40°C | 45.5 |
| Diffusion coefficient | 9.0 x 104 cm2 / sec. |
| Dipole moment, D | 4.3 |
| Evaporation rate index @ 25°C | |
| Relative to n-butyl acetate | 0.026 |
| Relative to diethyl ether | 0.0005 |
| Flammability limits in air | |
| lower (100°C) | 3 – 3.5% by volume |
| upper | 42 – 63% by volume |
| Flash point (open cup) | 95°C (203°F) |
| Flash point (closed cup) | 89°C (192°F) |
| Freezing point | 18.55°C (65.4°F) |
| Heat capacity, ideal gas, Cp(T°K) -0.227×10-4T2 | 6.94+5.6×10-2T |
| Heat capacity (liq.), 25°C | 0.47 cal / g / °C |
| Heat of combustion | 6054 cal / g |
| Heat of fusion | 41.3 cal / g |
| Heat of solution in water at 25°C | -54 cal/g |
| Heat of vaporization at 70°C | 11.3 kcal/mol |
| Henry’s constant @ 21°C | 991000 |
| Molar freezing point constant | 4.07°C / mol |
| Molar volume | 71.2 cm3 / g |
| Molecular weight | 78.13 |
| pKa | 35.1 |
| pK BH+ | -2.7 |
| Refractive index ND@25°C | 1.4768 |
| Solubility parameters | |
| Hansen’s | |
| – Dispersion | 9.0 (cal / cm3)1/2 |
| – Polar | 8.0 (ca l / cm3)1/2 |
| – Hydrogen bonding | 5.0 (cal / cm3)1/2 |
| Hildebrand’s | 13.0 (cal / cm3)1/2 |
| Specific heat at 29.5°C | 0.47± 0.015 cal /g /°C |
| Surface tension at 20°C | 43.53 dynes / cm |
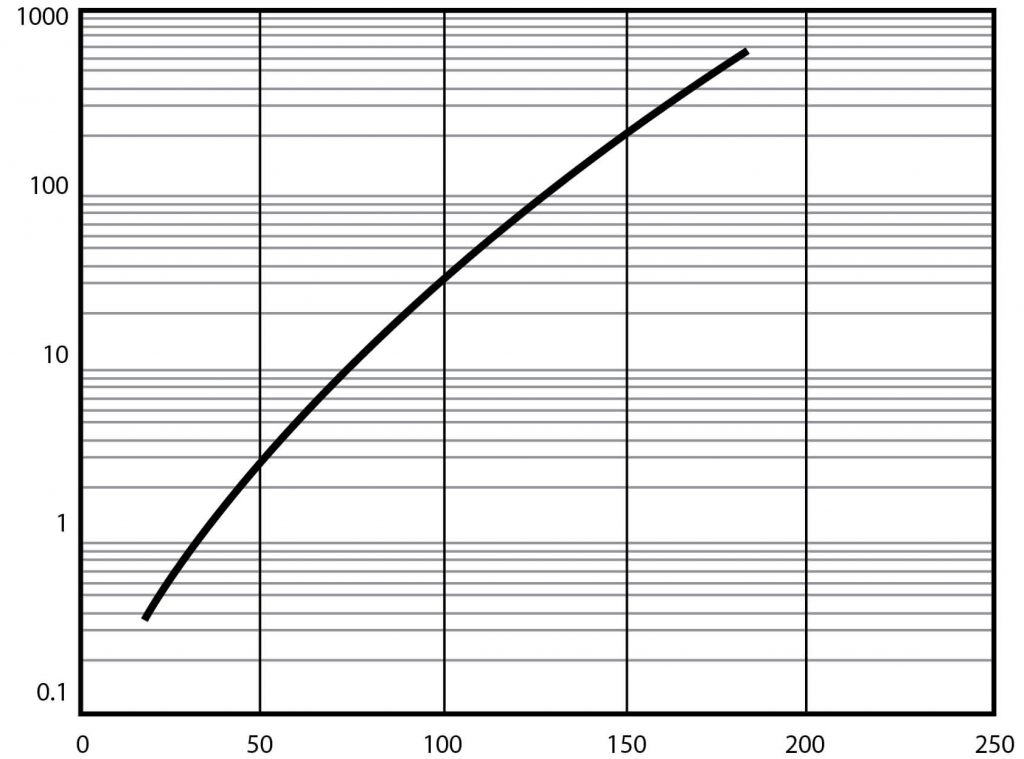
| Vapor pressure at 25°C (See Figure 1) | 0.600 mm Hg |
| Viscosity, cP, at 25°C (See Figure 4) | 2.0 |
| Log octanol-water partition coefficient | -1.35 |
Figure 4
DMSO Viscosity as a Function of Temperature
| Temperature (°C) | Viscosity (cP) |
| 25 | 1.991 |
| 30 | 1.808 |
| 40 | 1.511 |
| 50 | 1.286 |
| 75 | 0.916 |
| 100 | 0.691 |
| 125 | 0.546 |
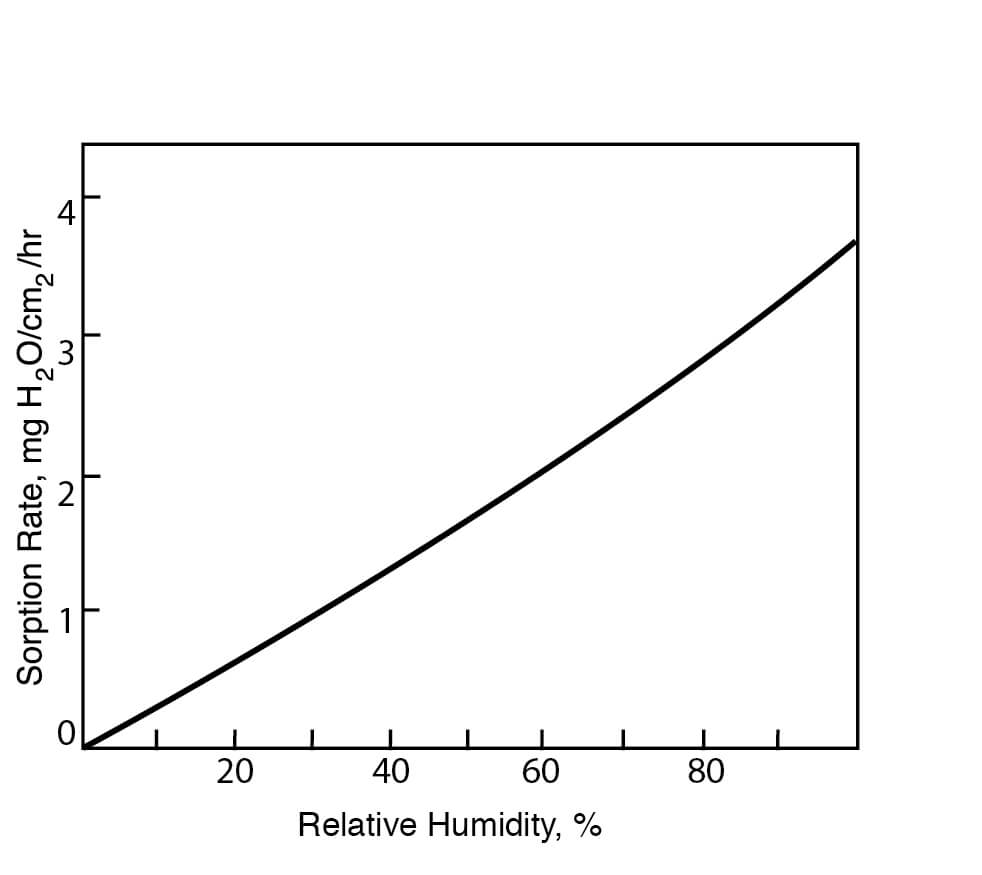
Figure 6
Hygroscopicities of DMSO at Various Relative Humidities at 22°C
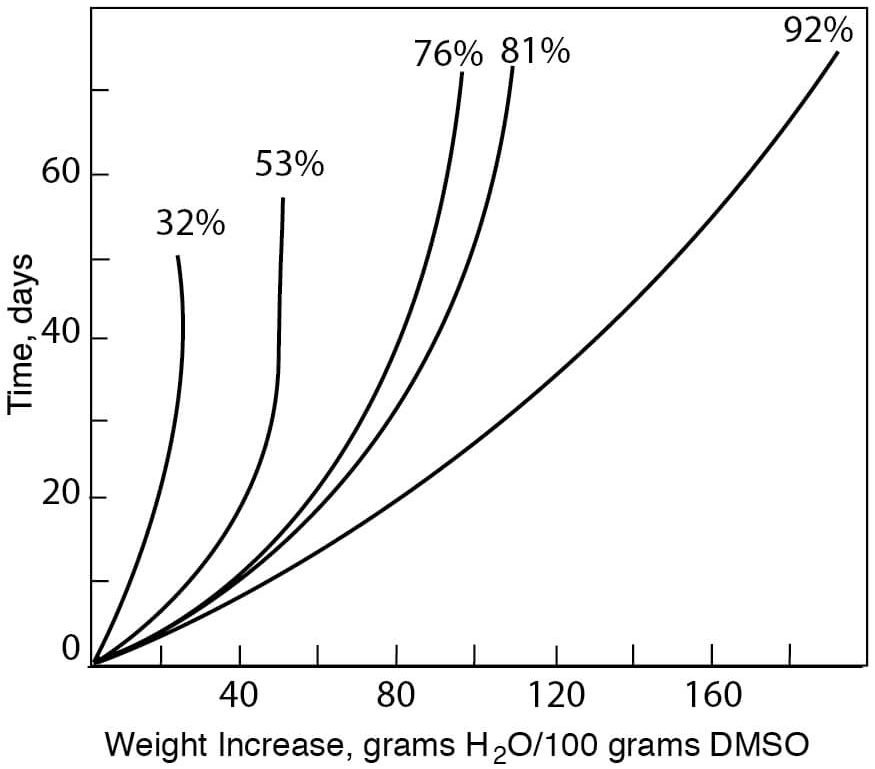
Figure 8
Freezing Point of DMSO Water Solutions
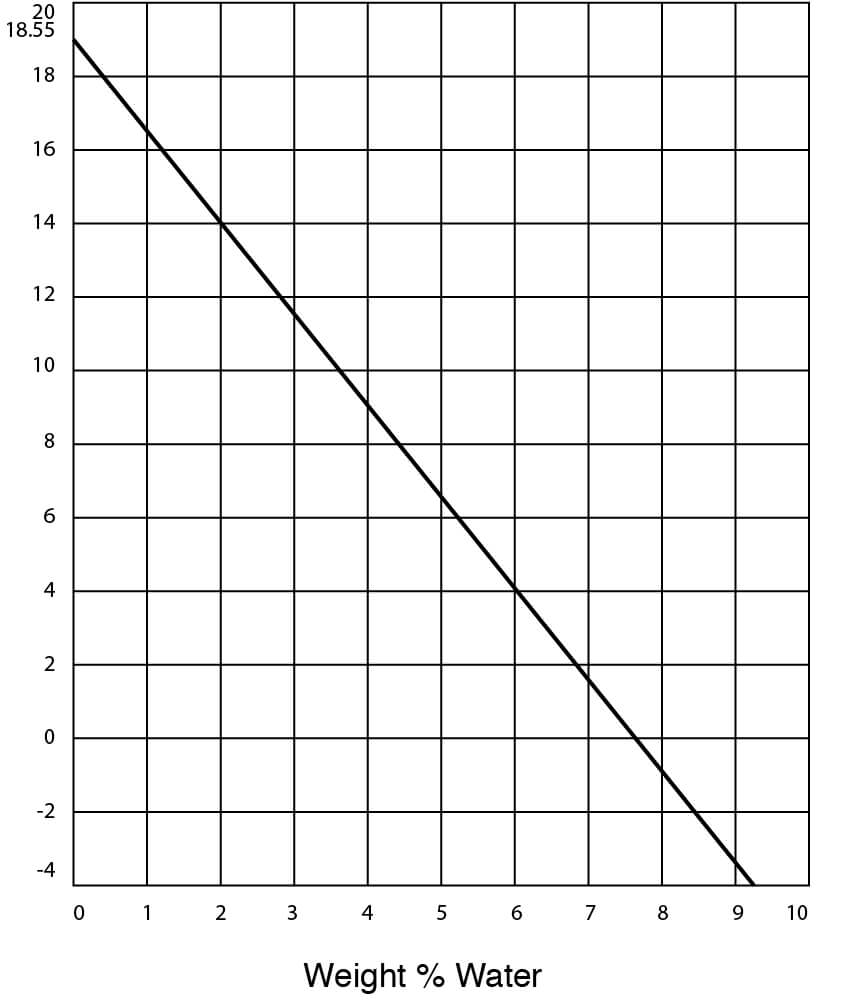
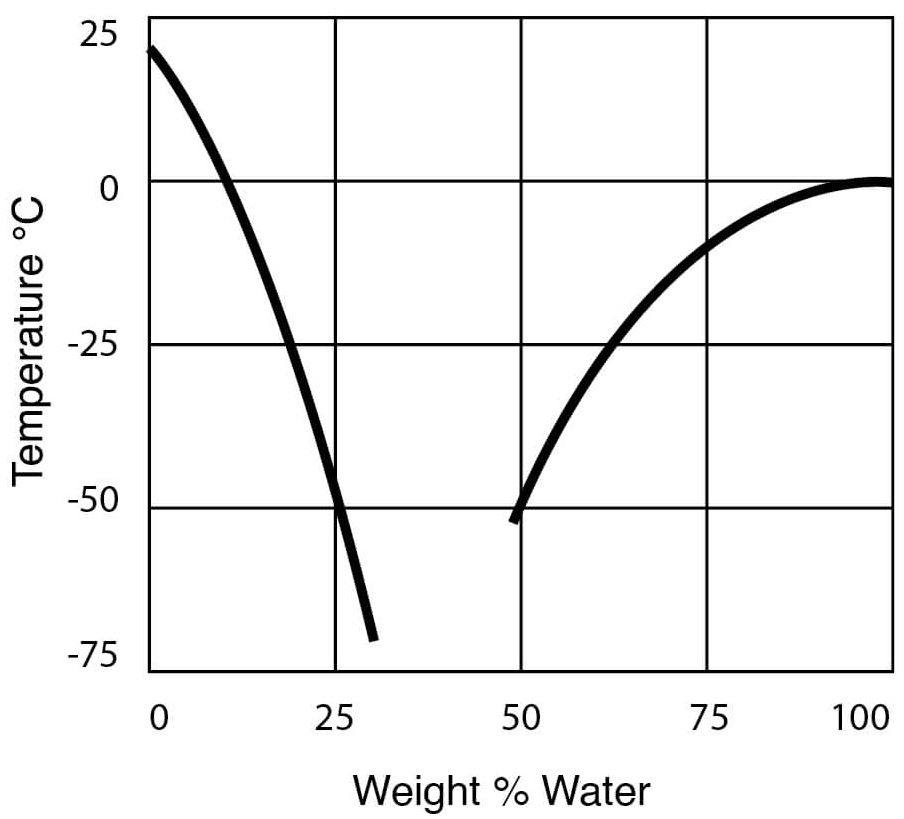
Figure 10
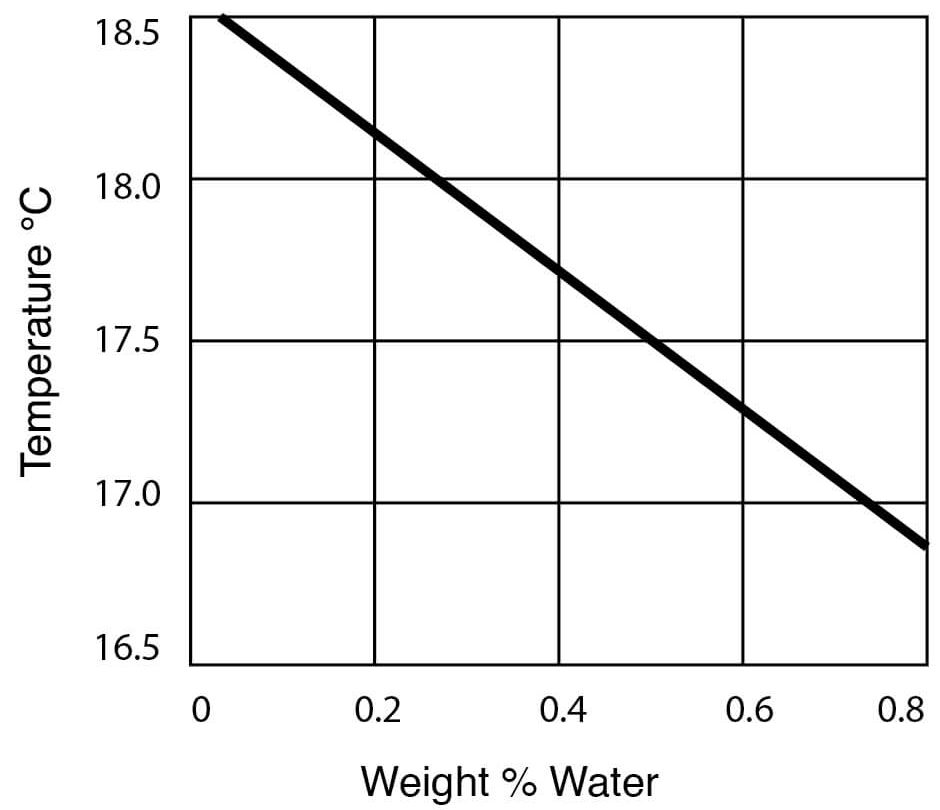
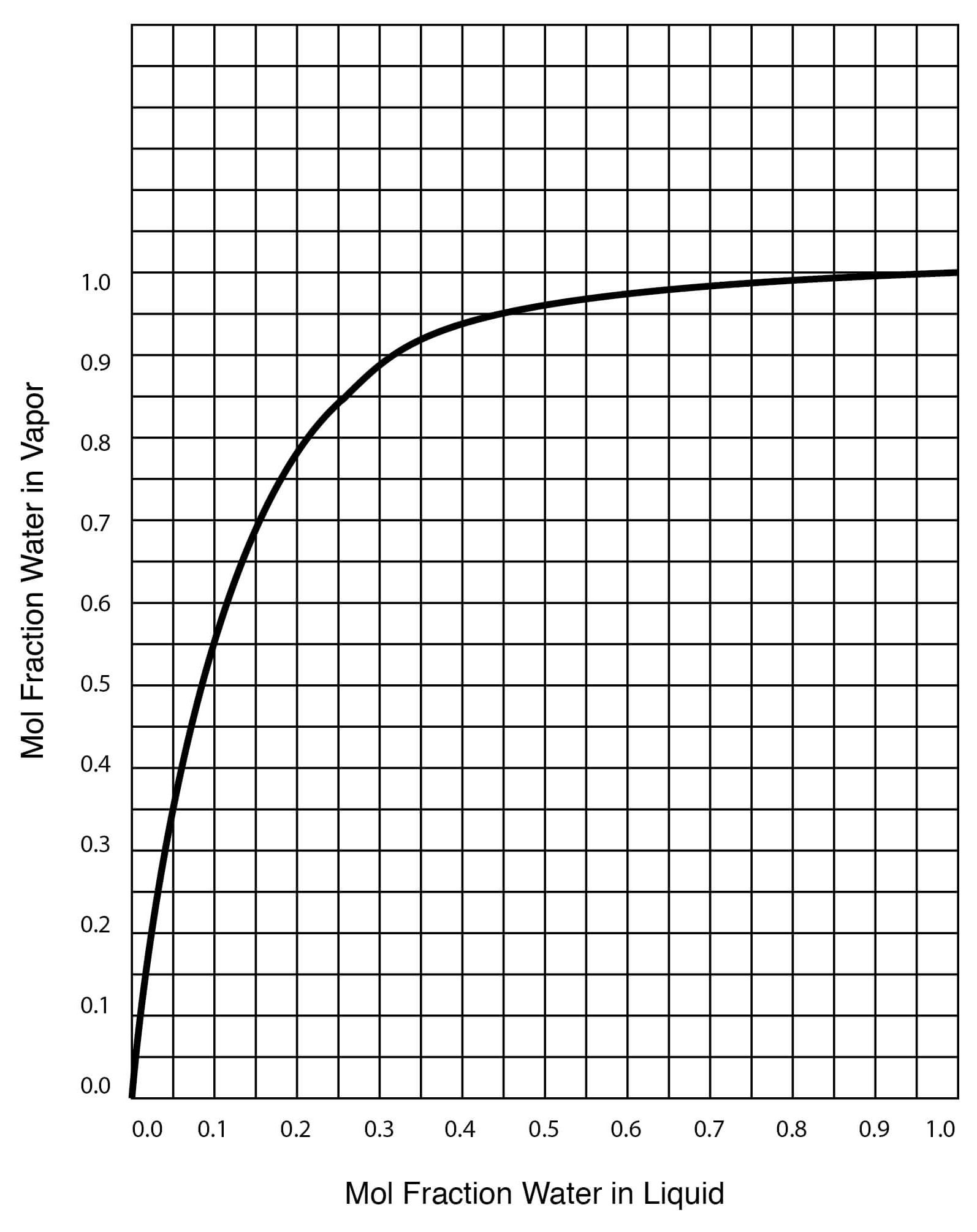
Figure 13
Vapor-Liquid Equilibrium for DMSO-Water Solutions (One Atmosphere Pressure)
| Temperature, °C | Mol Fraction Water in Liquid | Mol Fraction Water in Vapor |
| 100.0 | 1.000 | 1.000 |
| 100.6 | 0.988 | 0.9998 |
| 101.0 | 0.975 | 0.9997 |
| 102.0 | 0.945 | 0.9994 |
| 103.3 | 0.909 | 0.9989 |
| 105.0 | 0.865 | 0.9983 |
| 108.0 | 0.810 | 0.997 |
| 113.0 | 0.740 | 0.994 |
| 118.0 | 0.675 | 0.990 |
| 120.0 | 0.645 | 0.986 |
| 130.0 | 0.513 | 0.964 |
| 143.0 | 0.378 | 0.921 |
| 149.0 | 0.313 | 0.890 |
| 165.0 | 0.176 | 0.773 |
| 174.5 | 0.100 | 0.628 |
| 177.0 | 0.081 | 0.573 |
| 183.0 | 0.046 | 0.353 |
| 184.6 | 0.034 | 0.282 |
| 187.7 | 0.011 | 0.100 |
| 189.0 | 0.000 | 0.000 |
DMSO is highly stable at temperatures below 150°C. For example, holding DMSO at 150°C for 24 hours, one could expect a loss of between 0.1 and 1.0%. Retention times even in batch stills are usually considerably less than this, and therefore, losses would be correspondingly less. It has been reported that only 3.7% of volatile materials are produced during 72 hours at the boiling point (189°C) of DMSO. Slightly more decomposition, however, can be expected with the industrial grade material. Thus, about 5% DMSO decomposes at reflux after 24 hours. Al- most half of the weight of the volatile materials is paraformaldehyde. Dimethyl sulfide, di- methyl disulfide, bis-(methylthio)methane and water are other volatile products. A small amount of dimethyl sulfone can also be found. The following sequence of reactions explains the formation of these decomposition products:
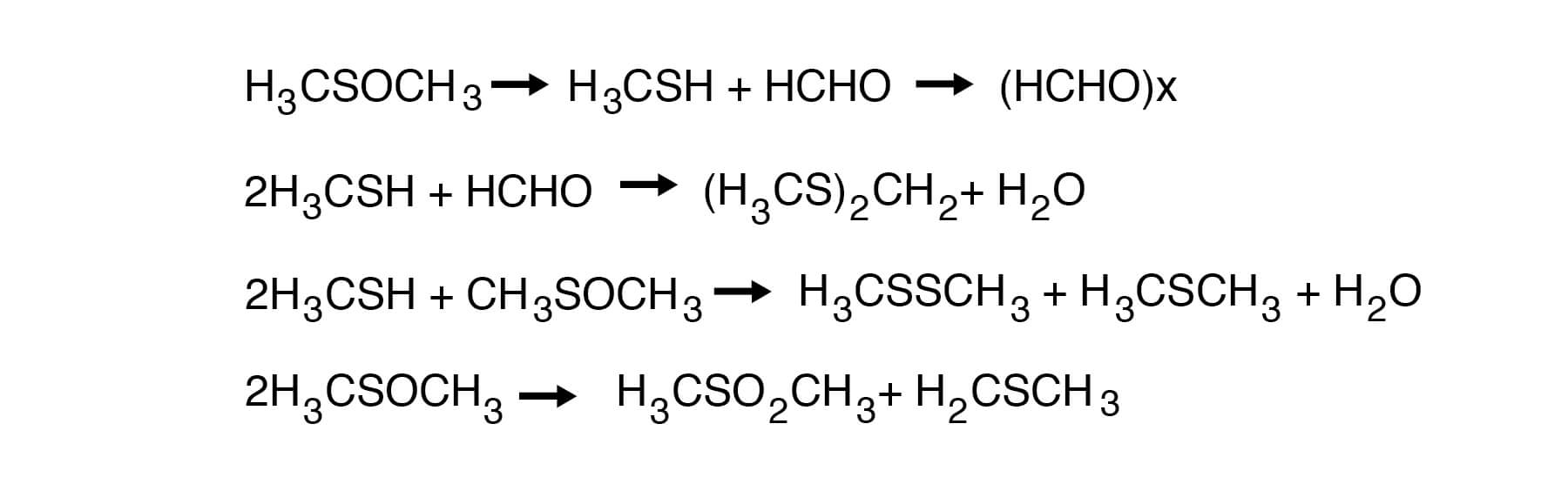
DMSO is remarkably stable in the presence of most neutral or basic salts and bases. When samples of DMSO (300g) are refluxed for 24 hours with 100g each of sodium hydroxide, sodium carbonate, sodium chloride, sodium cyanide, sodium acetate and sodium sulfate, little or no decomposition takes place in most cases. The results are shown in Figure 18.
Figure 18
Results of Reflux with DMSO and Various Materials (24 Hours)
| Compound (100g) in 300g DMSO | Reflux Temp. (°C) | DMSO Recovered % of Original | % Decomposition Products | ||||
| DMS(a) | DMDS(b) | BMTM(c) | HCHO(d) | MM(e) | |||
| NaOH | 185-140(f) | 93.7 | 63 | 31 | – | – | – |
| Na₂CO₃ | 190 | 96.3 | – | 14 | – | – | – |
| NaCl | 190 | 98.7 | – | 15 | – | – | – |
| NaCN | 148-164(g) | 100.0 | – | – | – | – | – |
| NaOAc | 182-187 | 97.0 | 22 | 33 | 8 | 20 | – |
| Na₂SO4 | 181-148(h) | 85.4 | – | – | – | – | – |
| DMSO Only | 189 | 98.0 | 15 | 30 | 30 | – | – |
| (a) Dimethyl sulfide (b) Dimethyl Disulfide (c) Bis-(methylthio)methane (d) Methyl mercaptan (e) Formaldehyde (f) Reflux temperature decreased from 185°C to 140°C over the first 16 hours (g) Reflux temperature was 148°C for 20 hours; increased to 164°C during the last 4 hours (h) Reflux temperature decreased gradually from 181°C to 148°C | |||||||
Figure 19
Thermal Stability of DMSO
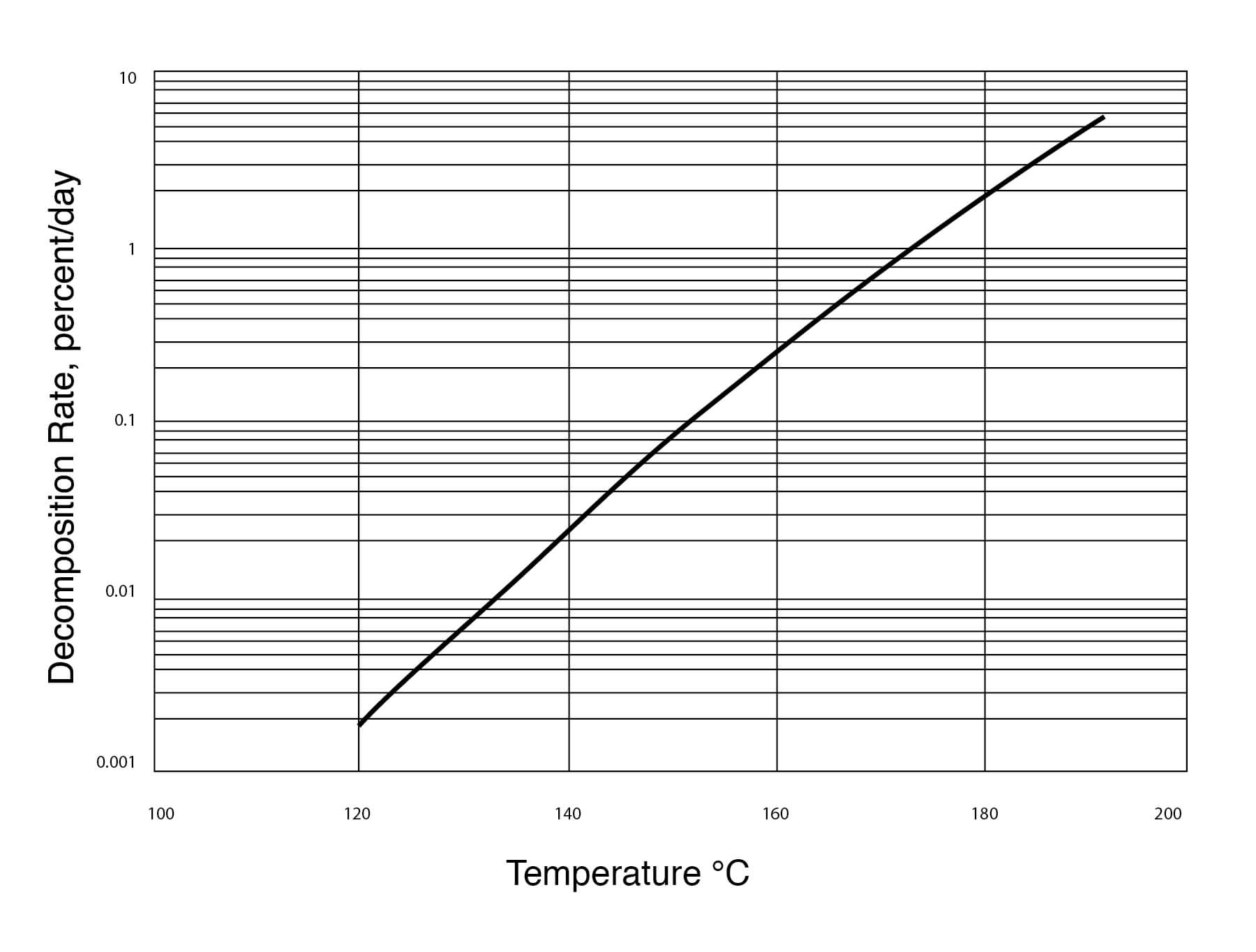
DMSO does not seem to be hydrolyzed by water and very little decomposition of DMSO takes place when it is heated under reflux for periods of 5 to 16 hours. The following tests, shown in Figure 20, have been performed: 1) 10 parts DMSO + 1 part water, 2) 60 parts DMSO + 5 parts water + 1 part sodium hydroxide, 3) 60 parts DMSO + 12 parts water + 1 part sodium bicarbonate, 4) DMSO alone.
Figure 20
Refluxing of DMSO and Mixtures, for Shorter Periods
| Composition of Sample Parts | Reflux Temp.(°C) | Time(hr) | Organic Products Composition, % | |||
| DMSO | DMS | DMDS | BMTM | |||
| 10 DMSO: 1 H₂O | 152 | 515 | 10099.7 | 00.15 | 00 | 00.15 |
| 60 DMSO: 5H₂O:1 NaOH | 155 | 58 | 99.899.3 | 0.10.6 | 0.10.1 | 00 |
| 60 DMSO: 12 H₂O: 1 NaHCO₃ | 131 | 612 | 99.999.8 | 0.10.2 | 00 | 00 |
| DMSO Only | 191 | 59
16 |
99.899.1
99.0 |
0.10.2
0.2 |
0.10.2
0.2 |
00.5
0.6 |
DMSO is also stable in the presence of concentrated sulfuric or hydrochloric acid at 100°C for up to 120 minutes of heating at atmospheric pressure. Phosphoric acid causes more rapid decomposition of DMSO than does sulfuric or hydrochloric acid. Detected decomposition products are dimethyl sulfide, dimethyl disulfide, and, in smaller quantity, formaldehyde. The results are shown in Figure 21:
Figure 21
Effect of Heating DMSO with Concentrated Acids
| Acid | Conc. | Temp.(°C) | Time(min.) | DMSO% left | % of decomposition product | ||
| DMS(a) | DMDS(b) | HCHO(c) | |||||
| H₂SO4 | 36% | 100 | 1530
120 |
9999
98 |
100100
100 |
||
| H₂SO4 | 36% | 125 | 15150
210 |
8686
80 |
77
10 |
9393
90 |
|
| H₃PO4 | 85% | 100 | 1530
45 60 120 150 |
9289
89 87 87 86 |
2545
45 46 46 50 |
7555
55 54 54 50 |
Some |
| H₃PO4 | 85% | 125 | 1560
150 |
8482
82 |
2533
33 |
7567
67 |
|
Figure 22
Thermal Decomposition of DMSO
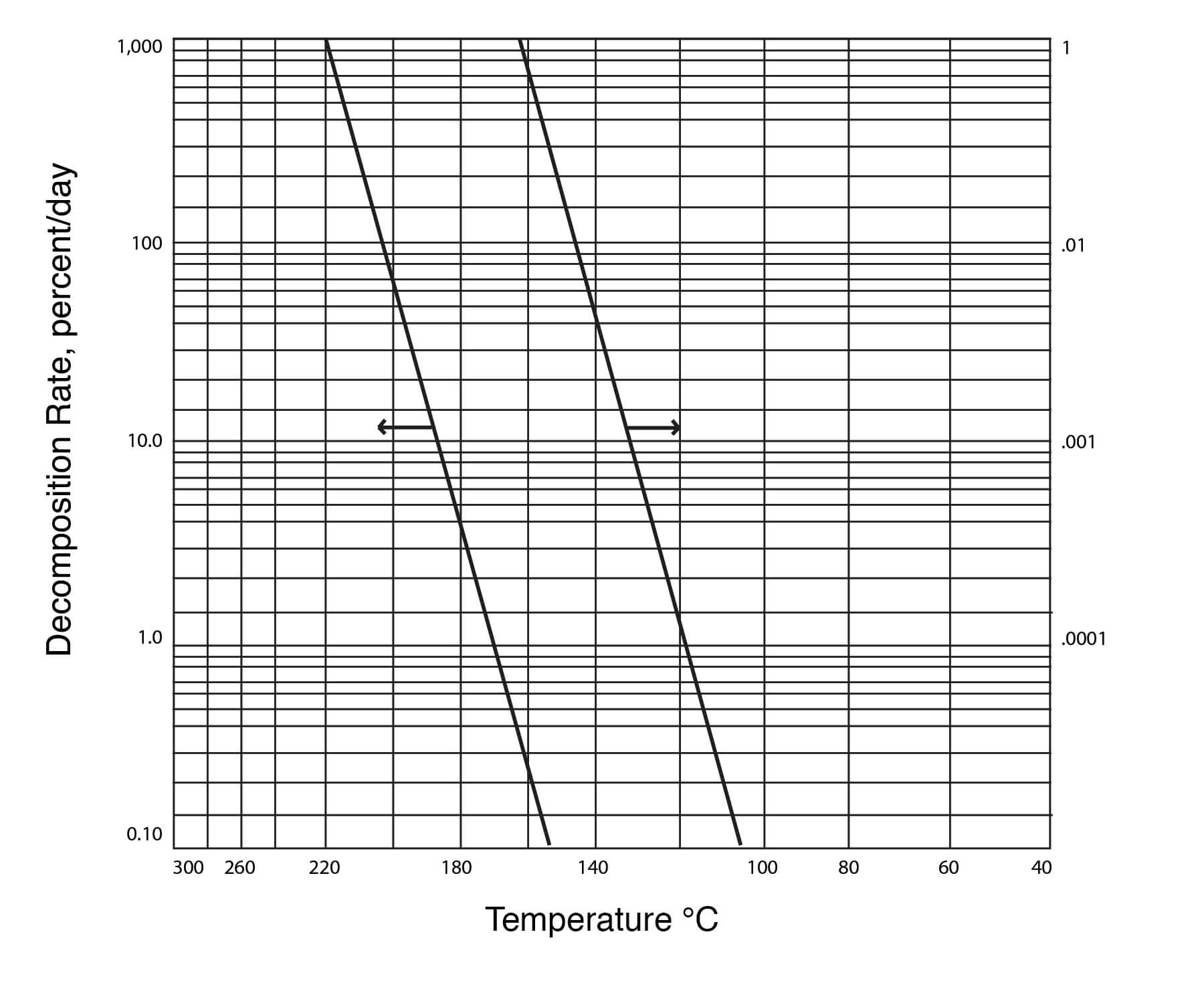
Figure 24
Comparative Evaporation Times for Common Organic Solvents
| Solvent | 90% Evaporation Times, seconds |
| Dimethyl Sulfoxide (DMSO) | 17,600 |
| Dimethylformamide (DMF) | 1,570 |
| N-Methyl-2-pyrrolidone (NMP) | 2,280 |
| Butyrolactone | 3,840 |
| Cyclohexanone | 15,400 |
| Isophorone | 20,000 |
| Diacetone alcohol | 23,700 |
| Propylene Carbonate | 119,660 |
| Sulfolane | >1,000,000 |
About Gaylord Chemical
The unique physical properties of DMSO makes it a useful solvent for a wide range of industrial applications. Gaylord Chemical has an extensive history as the preeminent producer of DMSO. We pride ourselves on delivering quality products, technical support, and world-class customer service. Locate a Gaylord Chemical distributor today.
Contact Gaylord Chemical Request a Sample
The information in this bulletin is based on information available to us and on our observations and experiences. However, no warranty is expressed or implied regarding the accuracy of this data, the results to be obtained from the use thereof, or that any use will not infringe any patent. Each user must establish appropriate procedures for off-loading, handling, and use of the product(s). Since conditions for use are beyond our control, we will make no guarantee of results, and assume no liability for damages incurred by off-loading, handling, or use of the product(s). Nothing herein constitutes permission, or recommendation to practice any invention covered by any patent without license from the owner of the patent.