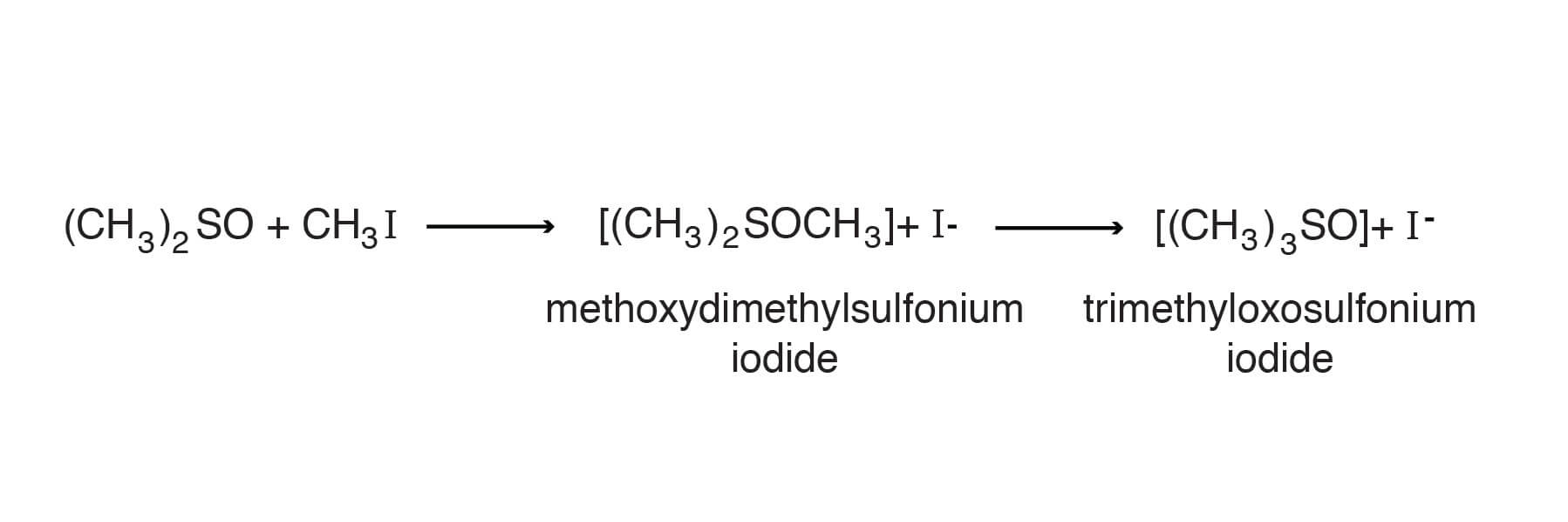
Alkylating agents, such as methyl iodide, react initially with DMSO at the oxygen to give methoxydimethylsulfonium iodide. These alkoxysulfonium salts are quite reactive and with continued heating rearrange to give the more stable trimethyloxosulfonium salts. In the case of methyl iodide trimethylsoxosulfonium iodide is produced [Smith, S. G.; Winstein, S.,Tetrahedron 3, 317-319 (1958)].
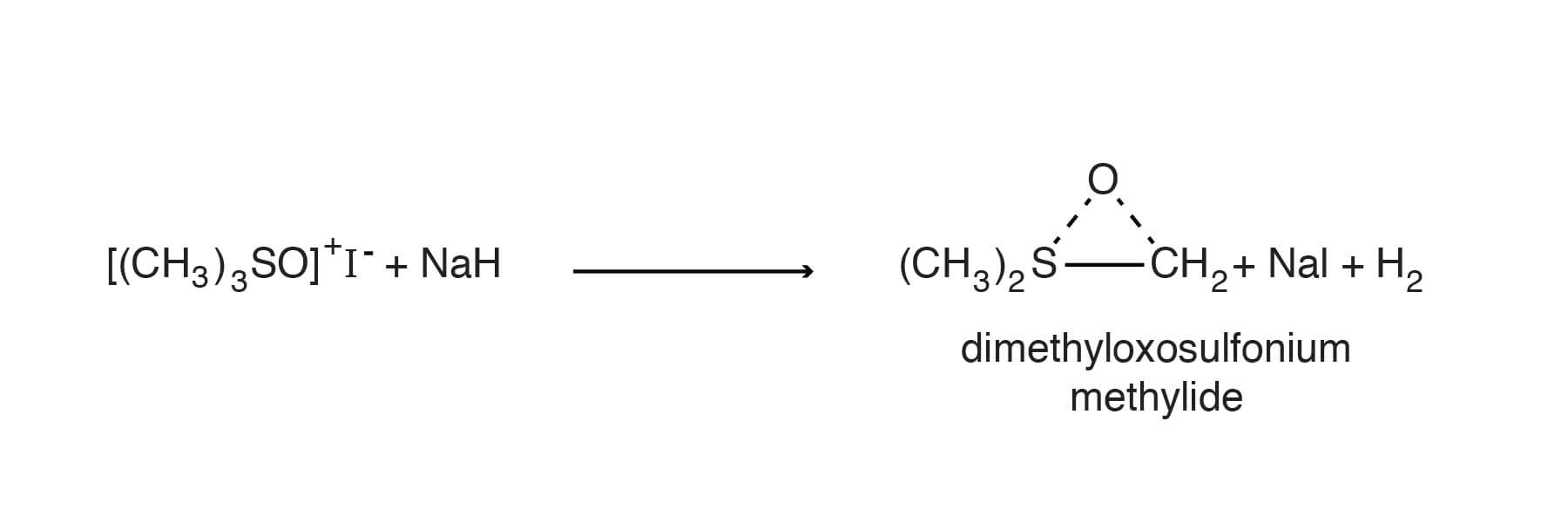
Trimethyloxosulfonium iodide is of interest because treatment with sodium hydride or dimsyl sodium produces dimethyloxosulfonium methylide which is an excellent reagent for introducing a methylene group into a variety of structures [Corey, E. J.; Chaykovsky, M., J. Am. Chem. Soc.87, 1353-1364 (1965); McCasland, G. E.; Naumann, M. O.; Durham, L. J. , J. Org. Chem.33, 4220-4226 (1968); zu Reckendorf, W. M.; Kamprath-Scholtz, U., Ber. 105, 686-695 (1972)].
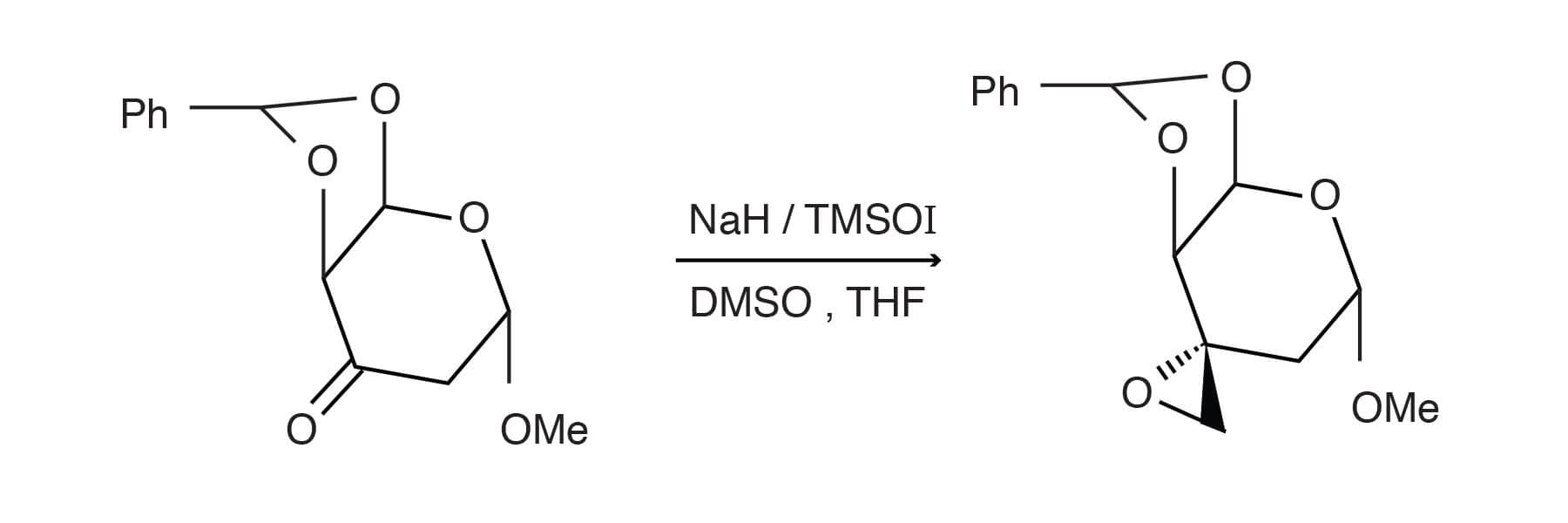
The following example, that employs dimethyloxosulfonium methylide (TMSOI), was described in Liebigs Ann. Chem., 1994, 999-1004.