Esterification is an important organic transformation which converts carboxylic acids (or their derivatives) into esters, via reaction with an appropriate alcohol. In some cases the alcohol itself may be employed as a reaction solvent, but many complex molecules require alcohols for which this approach is impractical. The alcohol may be too valuable to use in this manner, or may be a solid under the reaction conditions. When this is the case, DMSO is an excellent solvent choice. It often produces higher reaction yields with improved kinetics, relative to the other dipolar aprotic solvents.
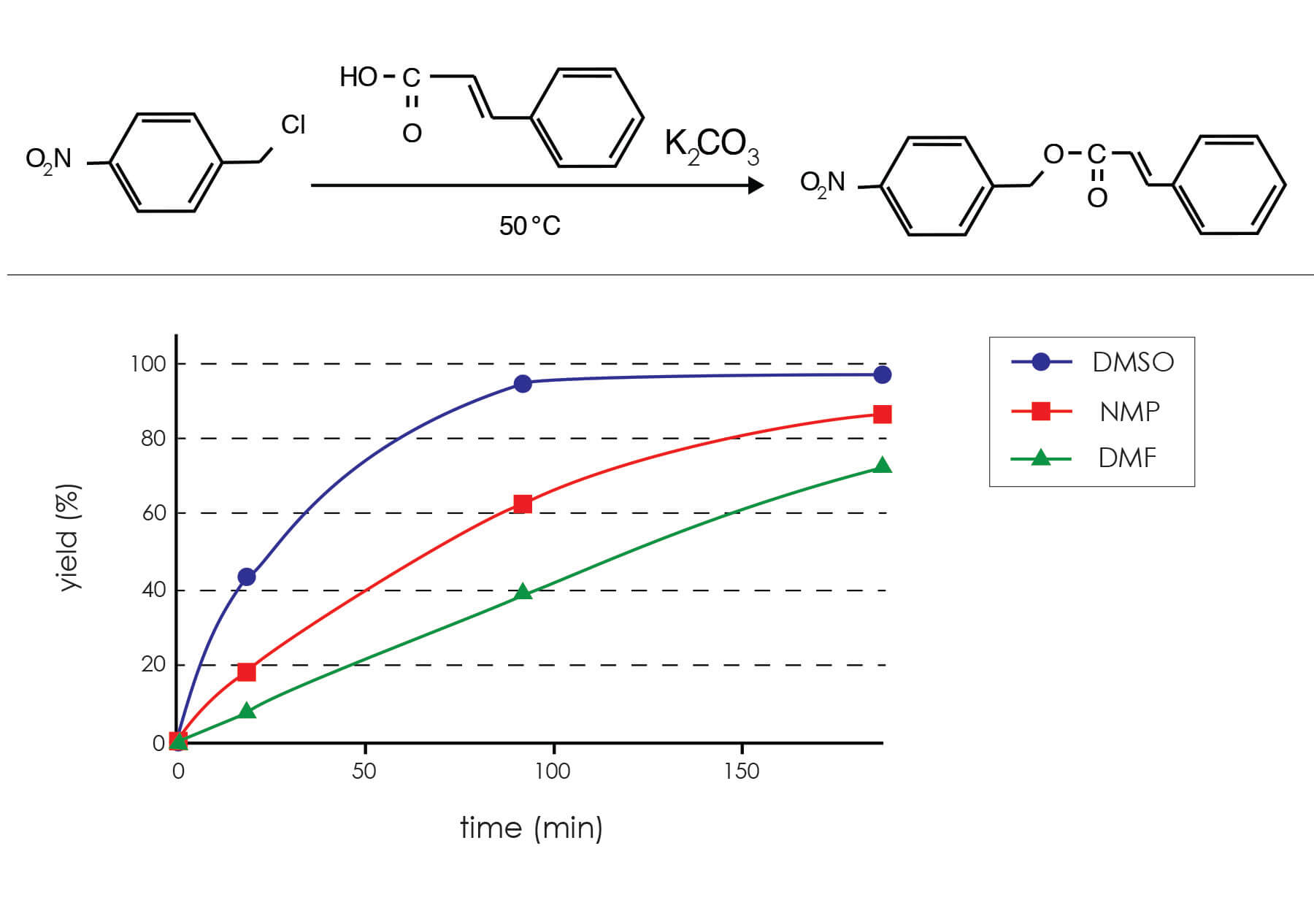
The base-catalyzed esterification reaction above demonstrates the important kinetic advantages often seen when DMSO as an esterification solvent. Not only is the product obtained in nearly quantitative yield in DMSO, but the reaction is complete in about half the time as with the other dipolar aprotic solvents tested.







