A number of organic compounds undergo hydrolysis, such as amides and esters. Ester hydrolysis may be acid or base catalyzed. Ester hydrolysis under basic conditions may be advantageously carried out with potassium hydroxide in aqueous DMSO.
Significant rate enhancements have often been reported for nucleophilic substitution reactions involving anionic nucleophiles as the solvent changes from H2O to DMSO. Such rate enhancements upon addition of DMSO to the reaction medium have been rationalized by postulates such as desolvation of the anionic nucleophile and/or stabilization of the transition state (TS). The negative end of the dipole of DMSO is exposed whereas the positive end is buried within the molecule. Therefore, DMSO stabilizes cations, whereas it strongly destabilizes anions due to the repulsion between the anion and the negative end of the dipole. It has generally been understood that destabilization of anionic species is more significant for small and charge localized anions than large and charge delocalized ones. Since earlier studies on ester hydrolysis have concluded that substrate solvation changes are not responsible for rate enhancements in DMSO, one can suggest that destabilization of OH– is largely responsible for the rate enhancement upon addition of DMSO to the reaction medium. [Um,I.-H.; Lee, E.-J.; Jeon, S.-E., Bull. Korean Chem. Soc. 2001, Vol. 22, 12 ,1301-2].
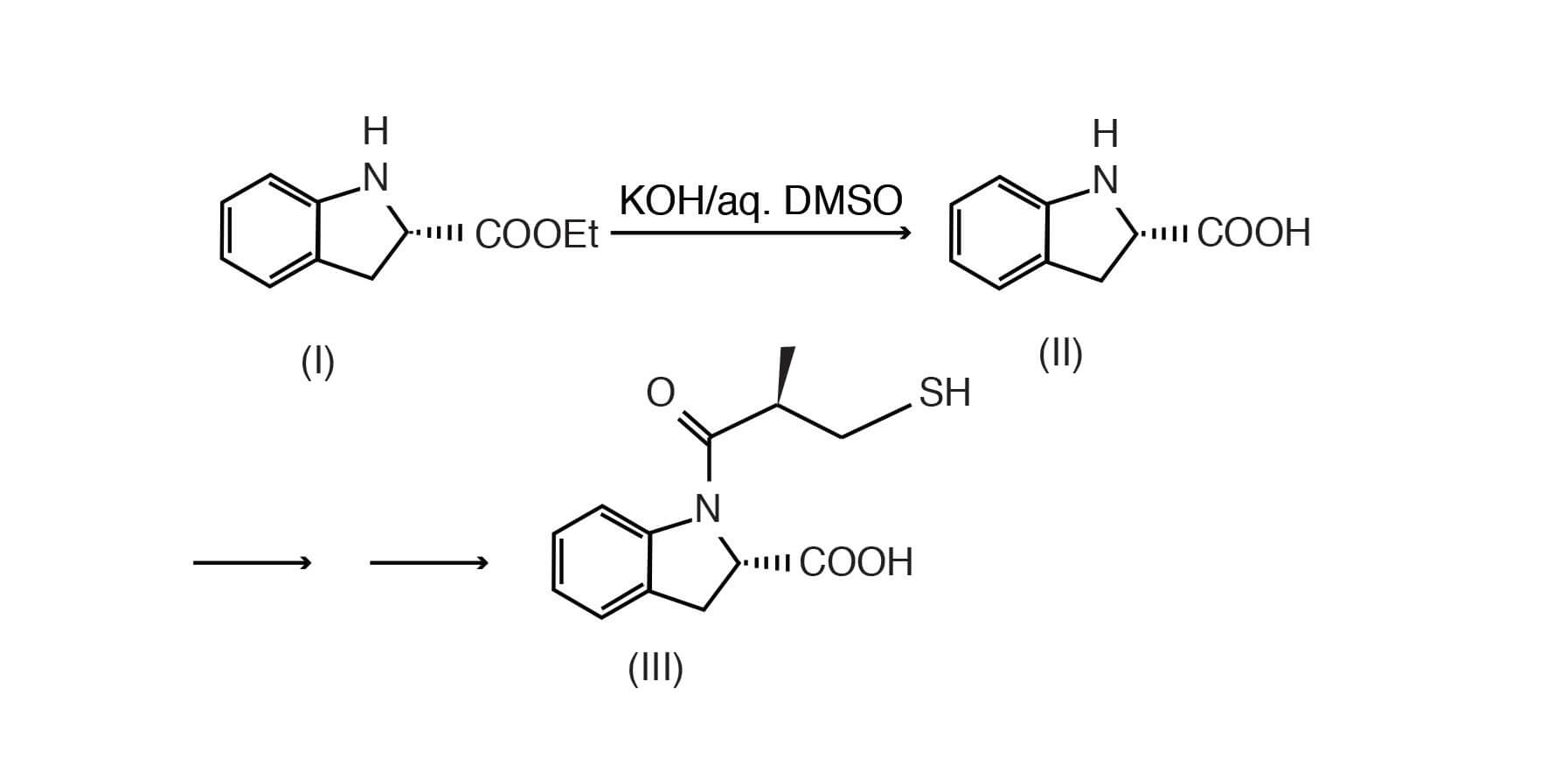
In the example below, the dihydroindole carboxylic acid ester was hydrolyzed to the free acid employing potassium hydroxide in aqueous DMSO, yielding an intermediate to an antihypertensive compound. [Stanton, J. L. et. Al., J. Med Chem, 1983 Sep; 26(9):1267-1277].