Quinolines are common structural units in pharmaceuticals and other biologically-active compounds.
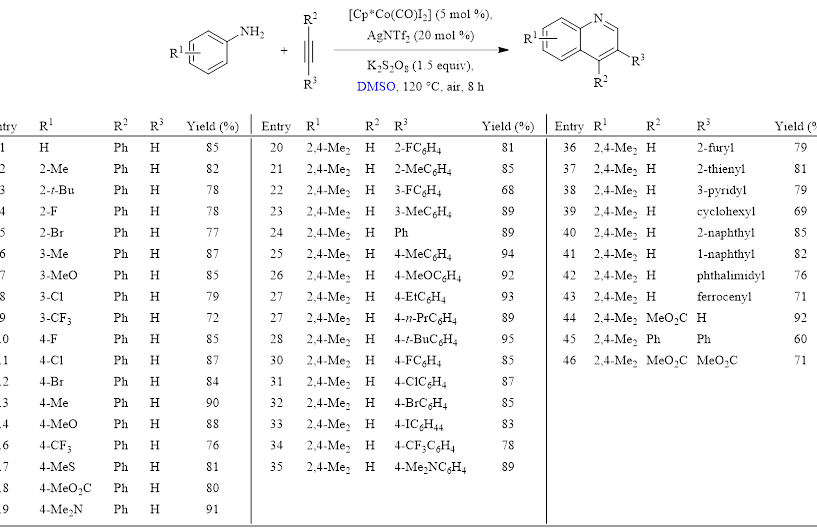
Table 1: Optimized Conditions and Substrate Scope
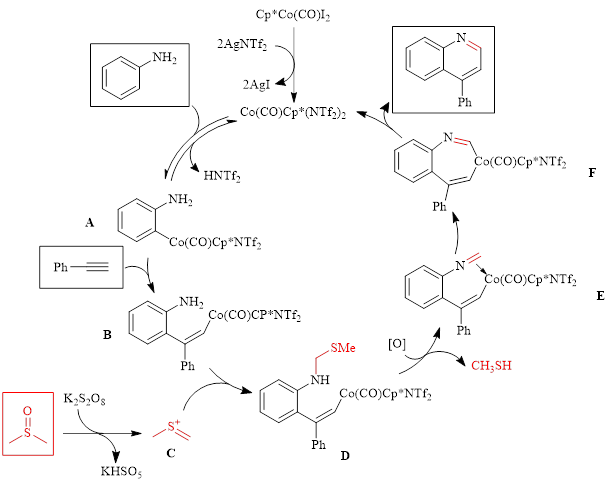
Mechanistic studies showed that DMSO serves as a one carbon synthon in the reaction as the use of DMSO-d6 results in a deuterium atom being located at the 2-position of the quinoline ring. With this knowledge, along with information from other mechanistic experiments, the authors proposed the mechanism shown in Scheme 1.
Scheme 1: Proposed Mechanism showing the role of DMSO as a C1 component
In this mechanism, the active catalyst is formed in situ by reaction of Cp*Co(CO)I2 with AgNTf2. Aniline then undergoes ortho metalation by the catalyst to form intermediate A, which then undergoes an insertion reaction with phenylacetylene to form intermediate B. Meanwhile, DMSO reacts with potassium persulfate to generate cation C, which undergoes nucleophilic attack by the amine to form intermediate D. Oxidation of intermediate D leads to the imine species E. Cobalt-carbon migratory insertion then results in intermediate F. Following protonolysis the product quinoline is formed, and the active cobalt catalyst is released.
This method by the Yi group shows the first example of a Co(III)/DMSO reaction using anilines and alkynes to form a variety of substituted quinolines. The method affords high yields with inexpensive and easily available starting materials. It also is mild, tolerating a wide array of functional groups. These attributes make this an attractive method to synthesize quinolines.
Debra D. Dolliver, Ph.D.
References
1 Xu, X.; Yang, Y.; Zhang, X.; Yi, W. Organic Letters 2018, 20, 566.