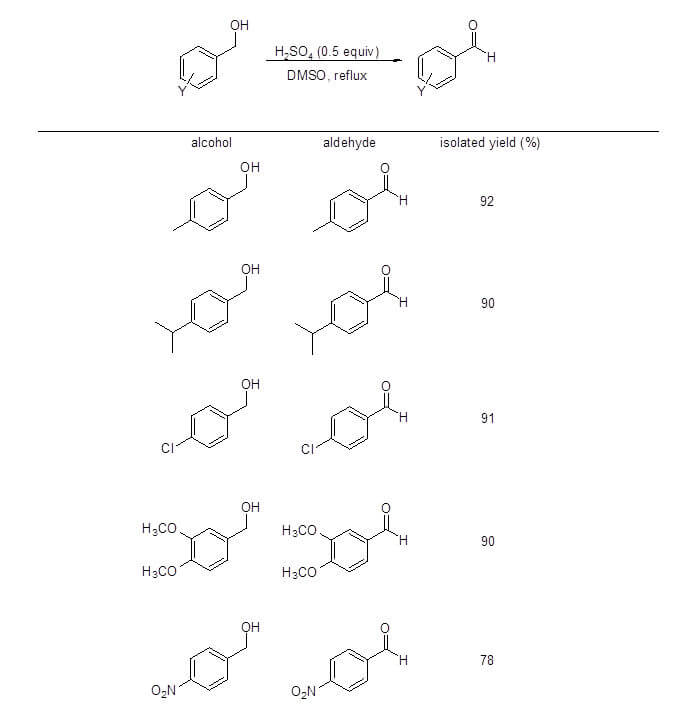
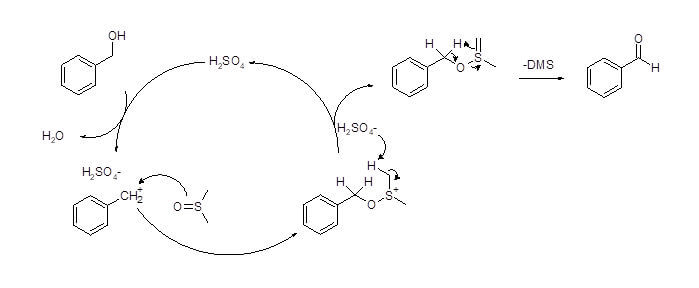
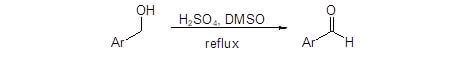DMSO is a useful reagent for selective oxidation of primary alcohols to aldehydes. These oxidations generally involve DMSO activation by another species such as dicyclohexylcarbodiimide [Pfitzner-Moffatt oxidation], oxalyl chloride [Swern oxidation] or pyridine sulfur trioxide [Parikh-Doering]. While these reactions have been widely used, they have some disadvantages in terms of atom economy, cost, difficult reaction conditions (low temperature, anhydrous atmosphere), toxicity, and unwanted byproducts. The newly reported procedure by Sheikhi’s group, however, eliminates many of these disadvantages and provides a low cost alternative procedure to convert benzylic alcohols to benzaldehydes using only sulfuric acid and DMSO (Figure 1).1