Novel polymers for DMSO-based In situ-forming implant systems

Although the above-listed polymers are quite popular, their in vivo administration can be associated with certain drawbacks; importantly, the accumulation of acidic degradation products generated during the hydrolysis of PLA and its derivatives2. Moreover, the control of drug release from these polymers tends to be especially challenging for hydrophilic macromolecules. Non-linear drug release profiles with high bursts have been observed3. Hence, there is interest in exploring the potential applications of novel polymers for the formation of DMSO-based implants. The results from three representative studies are summarized in this blog.
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) or PHBV, is a biodegradable, biocompatible, and water-insoluble polymer, suitable for in situ-forming implant systems. Hedayati et al studied in detail the development of a PHBV/DMSO based sustained delivery system for ariprazole, an antipsychotic drug4. Rheological measurements confirmed that the drug loaded PHBV-DMSO system forms a highly cross-linked network immediately upon exposure to aqueous buffer. Implants showed a biphasic pattern of drug release with an initial burst release followed by sustained drug release for 18 days. Drug release profiles were dependent on the initial polymer concentration in DMSO. Increasing polymer content led to the reduced burst release in the first 24 h, for example, 56% and 47% of cumulative drug release was observed with the polymer concentration of 2% and 4% w/v respectively. Interestingly, the overall cumulative drug release over an 18 day period was concomitantly reduced. Scanning electron microscopy (SEM) images showed that the porosity of the PHBV network increased significantly during the study period. The drug release from the PHBV-DMSO implant was compared with the carrageenan-based hydrogel system, which showed a comparatively higher burst drug release. . Thus, the PHBV/DMSO based in situ implant offers a novel formulation platform for the delivery of ariprazole.
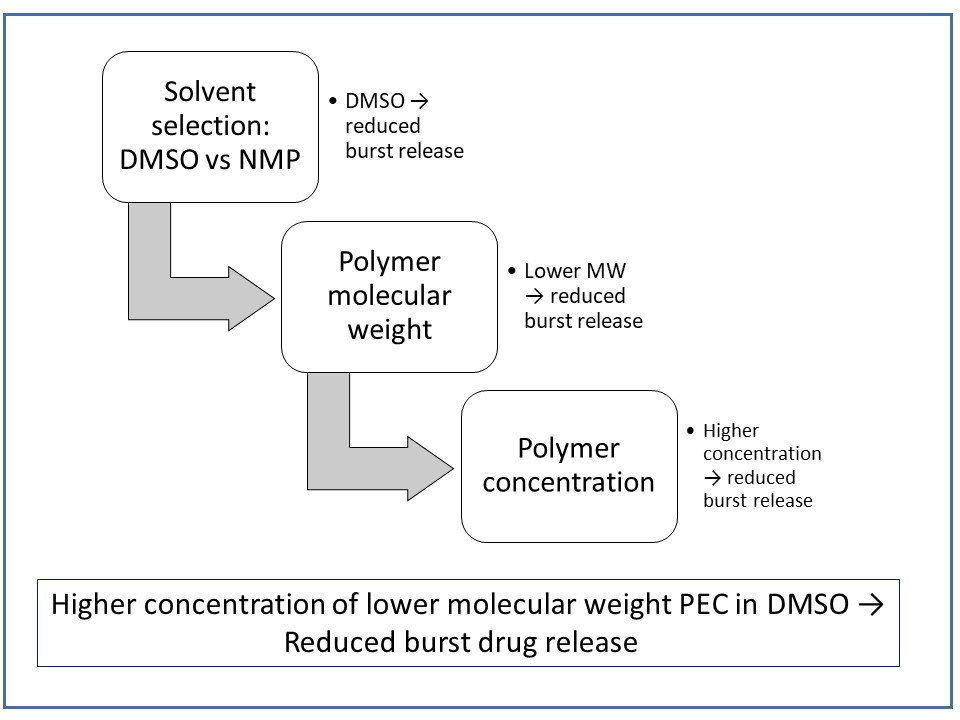
Poly(ethylene carbonate) or PEC, a carbonic acid derivative, is known not to degrade in vitro by hydrolysis at physiological pH. On the other hand, it degrades by oxidation in vivo through surface erosion. In this situation, the molecular weight of the polymer mass remains unchanged. Subcutaneous implants of PEC disappear within weeks to months from the body, with ethylene carbonate as the product of degradation5. Lin et al evaluated the feasibility of using PEC as a surface-eroding biomaterial for the preparation of biodegradable in situ forming parenteral drug delivery systems2. Different formulations of bovine serum albumin (BSA), the model drug, were prepared in PEC/DMSO or PEC/NMP solutions. A DMSO formulation of BSA displayed less burst drug release than a NMP formulation. The results from orthogonal techniques were used to explain the higher initial drug release from NMP-based implants. The SEM images of the depots formed using DMSO-based solution showed a less porous structure, as compared to theNMP formulation. Solvent release studies showed the faster release of DMSO from PEC depots relative to NMP, suggesting comparatively quick formation of the depots from DMSO solution. The faster formation of a semi-solid polymer layer on the surface of a depot can become an effective barrier to restrict mass transfer into the depot interior and slow the phase separation process. The authors propose that a solution of PEC in DMSO would be preferred over NMP due to the reduced burst release. In summary, these investigations demonstrate that in situ depot-forming systems can be obtained from PEC solutions.
Figure 1. A schematic approach to reduce burst drug release using PEC as polymer matrix. The approach is based on the summary of results obtained from the study by Lin et al2 and Chu et al6.
In a continuing study by the same research group, the effect of PEC polymer molecular weight on drug release from in situ-formed depots were investigated. A decrease in the polymer molecular weight from 200 kDa (PEC200) to 41 kDa (PEC41) led to significant decrease in the burst drug release. This was demonstrated by the release of 63.7% BSA from PEC200 in DMSO (15% w/w) in 2 days, as compared to 22.5% release from PEC41 samples. The burst drug release could be further reduced by increasing PEC41 concentration to 25% w/w; After 6 days, only 16.3% of drug was released. When compared to depots formed using PEC200, there was a slower rate of a solvent release, a slower phase inversion, and a denser surface in PEC41 samples. The authors conclude that the initial burst of the protein from the PEC41 depot was significantly reduced, which makes this a promising candidate polymeric carrier. A comprehensive strategy to modulate initial burst drug release based on the two studies is presented in Figure 1.
Seema Thakral, Ph.D.
References:
1.Thakur, R.R.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J Controlled Release. 2014, 76, 8-23.
2.Liu, Y.; Kemmer, A.; Keim, K.; Curdy, C.; Petersen, H.; Kissel, T. Poly (ethylene carbonate) as a surface-eroding biomaterial for in situ forming parenteral drug delivery systems: A feasibility study. Eur J Pharm Biopharm. 2010, 76(2), 222-229.
3.Körber, M.; Bodmeier R. Development of in situ forming PLGA drug delivery system, I: characterization of a non-aqueous protein precipitation. J. Pharm. Sci. 2008, 35, 283–292.
4.Hedayati, A.; G Yazdi, S.; Dehghankelishadi, P.; B Javan, N.; Akbari, H.; Dorkoosh, F. Preparation, optimization and physicochemical characterization of Aripiprazole loaded nano-porous in situ forming implant. Pharm Nanotech. 2017, 5(2), 138-147.
5.a Gunatillake, R. Adhikari, Biodegradable synthetic polymers for tissue engineering, Eur. Cell. Mater. 2003, 5, 1–16.
6.Chu, D.; Curdy, C.; Riebesehl, B.; Beck-Broichsitter, M.; Kissel, T. In situ forming parenteral depot systems based on poly (ethylene carbonate): effect of polymer molecular weight on model protein release. Eur J Pharm Biopharm. 2013, 85(3), 1245-1249.





Leave a Reply
You must be logged in to post a comment.