DMSO as the Oxidant in the α-Sulfenylation of Unsaturated Ketones
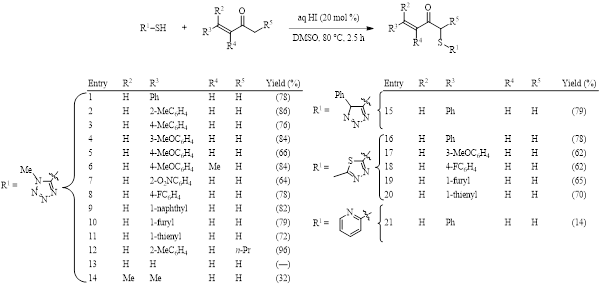
The number of reactions continues to grow where the interaction of DMSO and iodine result in the functionalization of a C–H bond. (See Synthesis Corner posts from July 28 & March 29, 2017 and October 29, 2016 for recent examples.) An example published in 2018 by the Prabhu group introduces the completely regioselective αʹ-sulfenylation of an α,β-unsaturated ketone using substoichiometric hydroiodic acid in DMSO (Table 1).1 This reaction is noteworthy in that it results in no conjugate addition, despite the presence of a nucleophilic thiol.
The optimized conditions shown in Table 1 were tested for substrate scope, giving good yields in all but a few cases (entries 13, 14, and 21). When the thiol contains a tetrazole group the yield is consistently good if there is an aromatic ring at the β position of the ketone (entries 1–12). When the thiol contains a triazole (entry 15) or a thiadiazole (entries 16–20), again, the yields are good.
Table 1. Optimized conditions and substrate scope.
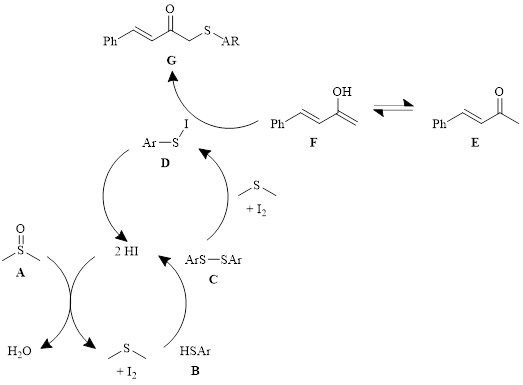
Mechanistic probes indicated that the reaction still produced the product in good yield in the presence of TEMPO, ruling out radical intermediates. Additionally, reactions beginning with the disulfide C (see Scheme 1) produced the product in good yield, indicating that the disulfide is an intermediate in the reaction. Starting the reaction with an αʹ-iodo α,β-unsaturated ketone (PhCH=CHCOCH2I) did not result in the sulfenylated product, indicating that the reaction does not proceed through the αʹ-iodo ketone. In the absence of DMSO, only the conjugate addition product was produced.
With these findings, the mechanism shown in Scheme 1 was proposed. In this mechanism, DMSO (A) interacts with HI to make dimethyl sulfide and iodine which oxidizes the thiol B to the disulfide C. The disulfide reacts with another equivalent of iodine to create the S–I bond in compound D. The iodo compound D reacts with the enolate ion F of the ketone E and produces the sulfenylated product G and regenerates HI.
Scheme 1: Proposed mechanism
This reaction represents a rare example where an α,β-unsaturated ketone does not undergo some sort of nucleophilic addition in the presence of a nucleophile. This reaction also represents a simple and mild method to functionalize an unsaturated ketone at the αʹ position. This work adds to the growing number of methods where DMSO and I2 work in concert to functionalize a C–H bond.
Debra D. Dolliver, Ph.D.
1 Siddaraju, Y.; Prabhu, K. R. The Journal of Organic Chemistry 2018, 83, 2986.







Leave a Reply
You must be logged in to post a comment.