DMSO as the carbon source in transition-metal-free α-Csp3-H methylenation
The ability to make the C=C bond at a targeted position has always been of high interest to synthetic chemists. Recently, Guo et al. reported a method by which to activate a hydrogen at the alpha position of a ketone in order to make a new C=C bond at that position (Figure 1).1 Their method requires no metal catalyst, uses readily-available starting materials, and requires no exotic reagents. DMSO is critical in this reaction as it acts as the methylene source.
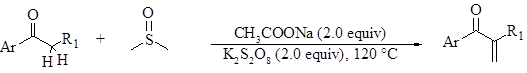
Figure 1: DMSO as the one-carbon source in the α-methylenation of ketones.
Figure 1 shows the optimized conditions for the reaction. Perfomance of the reaction with a variety of substituents attached to the aromatic ring (electron-withdrawing to electron-donating) at the 2, 3, and 4 positions, gave yields that were generally good. (The 4-bromo substrate gave a yield of 42% which was the only example with a yield below 50%.)
These researchers also varied the nature of R1, and they found that the reaction was also tolerant of changes at this position. For example, changing R1 from a methyl to a propyl group changed the yield from 72% to 67%, respectively.
This work also explored the mechanism of the reaction. They showed that the addition of radical inhibitors did not significantly change the outcome of the reaction. This indicated that the reaction does not proceed through a radical pathway. They also performed the reaction starting with ketones with oxidized alpha carbons (α-aldehyde and α-carboxylic acid). These reactions yielded none of the methylenated product. This implied that the ketone does not undergo oxidation at the alpha position during the course of the reaction. When they started the reaction from the thiomethyl compound C, it proceeded smoothly to the product. This was a strong indication that compound C is formed during the reaction. Additionally, they ran the reaction with deuterated DMSO which resulted in the addition of =CD2 at the alpha position.
With these findings, the mechanism seen in Figure 2 was proposed. The ketone containing alpha hydrogens undergoes deprotonation to form the enolate ion (A). Simultaneously, DMSO interacts with potassium persulfate to form the ion B. Ion B undergoes nucleophilic attack by A to form compound C. Further reaction of compound C with the potassium persulfate results in loss of methanethiol and formation of the carbon-carbon double bond.
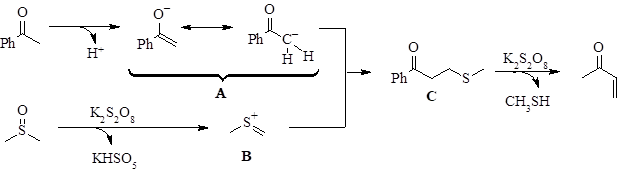
Figure 2: Proposed mechanism.
In conclusion, these researchers have developed a metal-free method to make a new C=C bond at the alpha position of a ketone. The method has wide substrate scope and uses readily available reagents. DMSO plays an important role in the reaction, as it provides the electrophilic methylene group that undergoes nucleophilic attack by the enolate carbon. This reactive methylene source is readily provided by reaction of DMSO with potassium persulfate.
Debra D. Dolliver, Ph.D.
References
- Liu, Y.-F.; Ji, P.-Y.; Xu, J.-W.; Hu, Y.-Q.; Liu, Q.; Luo, W.-P.; Guo, C.-C., Transition Metal-Free α-Csp3-H Methylenation of Ketones to Form C═C Bond Using Dimethyl Sulfoxide as Carbon Source. J. Org. Chem. 2017, 82 (14), 7159-7164.



