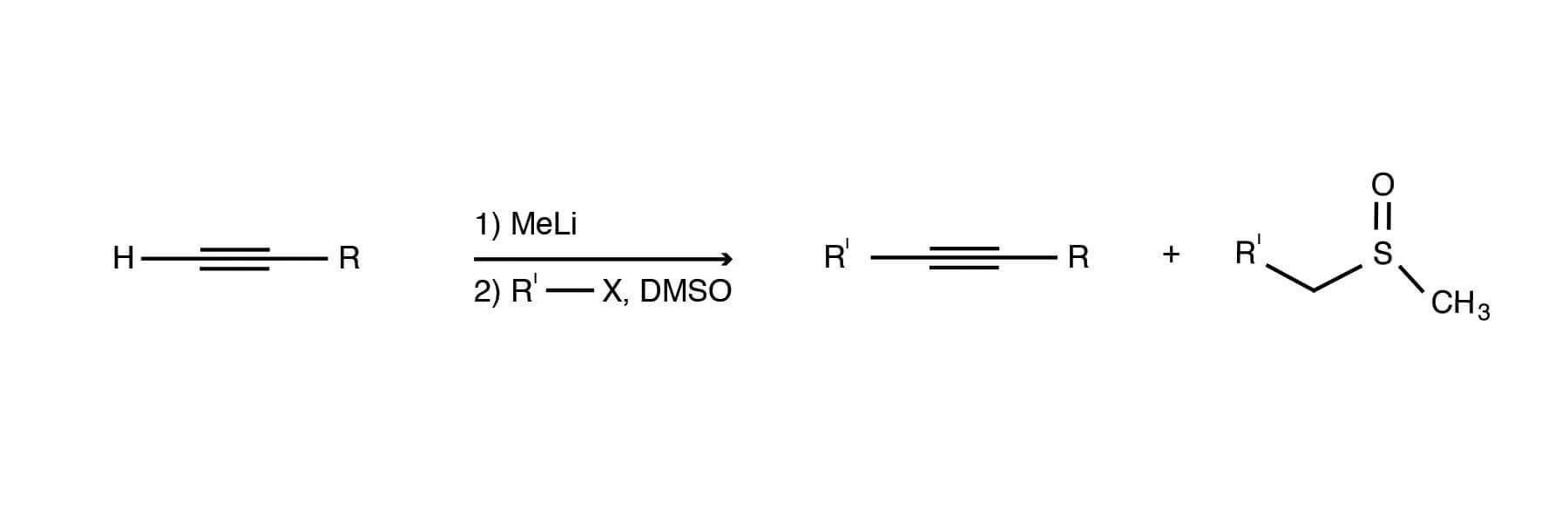
DMSO facilitates the N-alkylation of amides. The reaction of various haloamides with the dimsyl ion in DMSO can be used to obtain good to high yields of 4-, 5- and 6-membered lactams. However, this reaction fails to produce the seven-membered heterocyclic ring [Michael Chong, Susanna Wong, Alkylation of stabilized acetylides in DMSO. Preparation of α,β-acetylenic alcohols and acetals., Tetrahedron Letters, Volume 27, Issue 45, 1986, Pages 5445-5448, ISSN 0040-4039, https://dx.doi.org/10.1016/S0040-4039(00)85233-8.]. It is likely that entropic effects work against the formation of ring sizes larger than six.
X = Br, Cl, F, I;
N = 2, 3, 4
The dimsyl ion also adds to carbon-carbon double bonds, and if the mixture is heated for several hours, the initial adduct eliminates methanesulfenic acid. The overall result is methylation and with compounds such as quinoline or isoquinoline, yields are nearly quantitative [Russell, G. A.; Weiner, S. A., J. Org. Chem. 31, 248-251 (1966)] .
Care is required in running these reactions because the decomposition of the intermediate sulfoxide anion (and also dimsyl sodium) during the heating in the strongly alkaline system is exothermic.