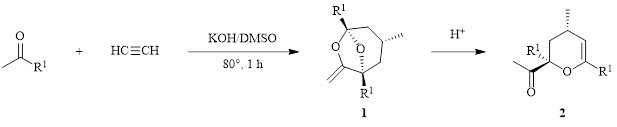
The number of self-assembly reactions involving acetylenes under KOt-Bu– and KOH–DMSO superbase conditions continues to grow.1 The Trofimov group has shown that simple ketones and acetylene can self-assemble in a one-pot KOH–DMSO reaction to provide 6,8-dioxabicyclo[3.2.1]octanes (DOBCOs) (1), which, upon treatment with acid, isomerize to the corresponding acetyl-substituted dihydropyrans 2 (Scheme 1).2 There is interest in species like compound 1 as this structural motif is abundant in many natural product compounds.
DMSO–Superbase Route to Dioxabicyclooctanes from Simple Ketones and Acetylenes
Scheme 1: KOH–DMSO superbase synthesis of an acetyl-substituted dihydropyran 2.
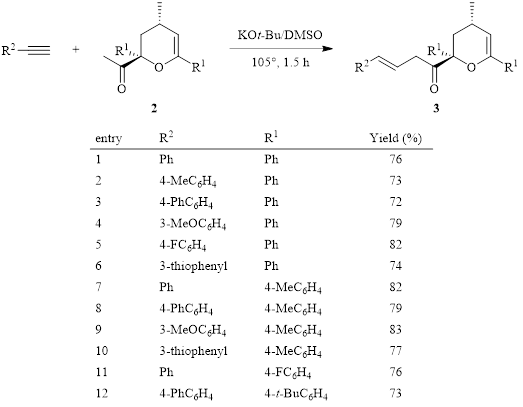
Recently, further elaboration by DMSO–superbase-promoted C-vinylation of the acetyl group of compound 2 by reaction with an aromatic alkyne has also been demonstrated by the Trofimov group (Table 1).3 This reaction appears to be only mildly affected by the electronic character of the aromatic alkyne, with all akynes in Table 1 resulting in similar yields. Of note, if R1 is a large aromatic group (i.e., PhC6H5 or 2-naphthyl), the C-vinylation reaction fails, and only deacetylation of the dihydropyran ring occurs.
Table 1: KOt-Bu–DMSO superbase C-vinylation of acetyl-substituted dihydropyran 2.
The Trofimov group also developed a cyclization method whereby compound 3 is converted into the dienyl DOBCO 4 (Scheme 2).3 In other words, following a self-assembly reaction between acetylene and a simple ketone (Scheme 1), subsequent C-vinylation with another alkyne (Table 1), and then cyclization (Scheme 2), functionalized DOBCOs like compound 4 can be readily synthesized through the two highlighted DMSO superbase reactions.
Scheme 2: Cyclization of dihydropyran 3 to DOBCO 4.
This route is transition-metal-free, requires only readily-available starting materials, and proceeds through simple conditions and protocols.
Debra D. Dolliver, Ph.D.
1 Trofimov, B. A.; Schmidt, E. Y. Accounts of Chemical Research 2018, 51, 1117.
2 Trofimov, B. A.; Schmidt, E. Y.; Ushakov, I. A.; Mikhaleva, A. I.; Zorina, N. V.; Protsuk, N. I.; Senotrusova, E. Y.; Skital’tseva, E. V.; Kazheva, O. N.; Alexandrov, G. G.; Dyachenko, O. A. European Journal of Organic Chemistry 2009, 2009, 5142.
3 Schmidt, E. Y.; Tatarinova, I. V.; Semenova, N. V.; Protsuk, N. I.; Ushakov, I. A.; Trofimov, B. A. The Journal of Organic Chemistry 2018, 83, 10272.