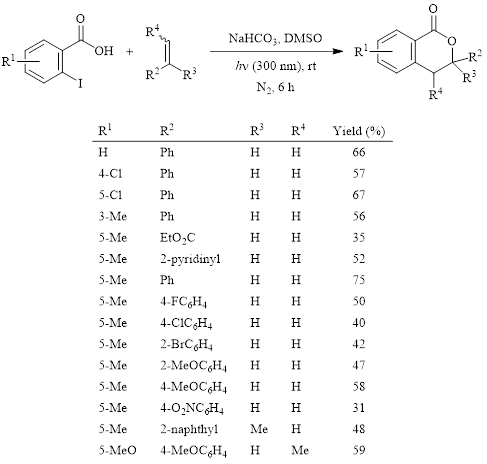
Isocoumarins exhibit a wide range of biological activity. Therefore, they are often synthetic targets. Many of the recently developed methods to form the isocoumarin or 3,4-dihydroisocoumarin structure rely on metal catalysts and relatively high temperatures. In 2018, Xia and Yang’s group reported a light-promoted method in DMSO to form substituted 3,4-dihydroisocoumarins.1 This method does not require expensive metal catalysts and/or ligands or elevated temperatures. The optimized conditions and the substrate scope are shown in Table 1. During the optimization of this reaction, it was found that using DMSO as the solvent afforded superior yields.
Light-Promoted Synthesis of 3,4-Dihydroisocoumarin Derivatives in DMSO
Table 1: Photocatalyzed synthesis of 3,4-isocoumarin derivatives in DMSO.
The reaction resulted in moderate yields in all cases. Electronic effects of substituents are modest, so this reaction is effective with both electron donating and electron withdrawing groups on either the iodobenzoic acid ring or on the alkene.
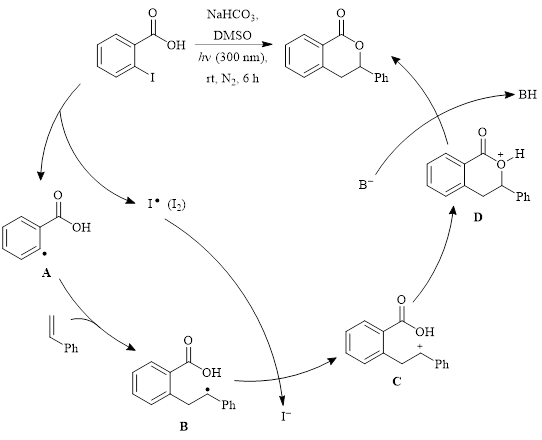
Mechanistic studies revealed that the addition of TEMPO to the reaction resulted in a yield of only 5%, suggesting that the mechanism involves radicals. The authors proposed the mechanism shown in Scheme 1 for this transformation. In this process, dehalogenation produces the aryl radical A which adds the alkene to produce radical intermediate B. Intermediate B reduces I2 to I– which creates the carbocation C. Cyclization to produce D, followed by deprotonation, creates the 3,4-dihydroisocoumarin product.
Scheme 1: Proposed mechanism.
In summary, this reaction provides a route to 3,4-dihydroisocoumarins that does not require an expensive metal catalyst. It is run at room temperature with readily available reagents. These attributes make it an attractive option for synthesizing this valuable structural unit.
Debra D. Dolliver, Ph.D.
1Zhang, X.; Huang, B.; Yang, C.; Xia, W. Synlett 2018, 29, 131-135.