What is Elimination?
Eliminations are base-catalyzed reactions in which two atoms or groups are removed or eliminated, usually from one or two carbon atoms. A double bond is frequently formed as the result of this elimination.
The pyrolysis of t-amine oxides (Cope elimination) in dry DMSO proceeds at a convenient rate at 25°C to give 80- 90% yields of olefins. Temperatures of 132-138°C are usually required in water. In addition, the rates in DMSO are 10,000 times faster than in water [Cram, D. J.; Sahyun, M. R. V.; Knox, G. R., J. Am. Chem. Soc. 84, 1734-1735 (1962)].
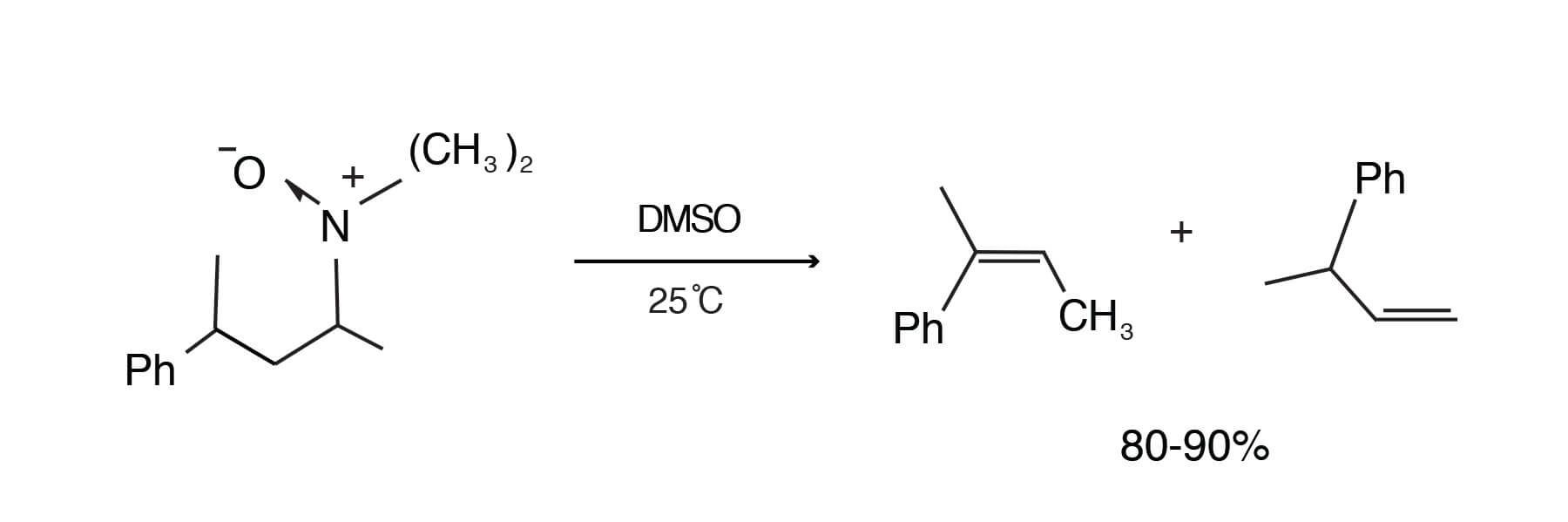
The rate is higher in wet DMSO than in dry THF because DMSO acts as an internal drying agent and competes with amine oxide for the water present [Sahyun, M. R. V.; Cram, D. J., J. Am. Chem. Soc. 85, 1263-1268 (1963)].
Pure olefins from their dibromides can be obtained by using sodium thiosulfate in DMSO as the debrominating agent. Thus, stilbene dibromide yields stilbene [Ibne-Rasa, K. M.; Tahir, A. R.; Rahman, A., Chem. Ind. (London) 232 (1973)].

